For your patients 1 year of age and older with type 1 diabetes
Tresiba® (insulin degludec) provides proven A1C reduction with once-daily dosing1
Efficacy and safety evaluated in adults with T1D
Safety study in adults with T1D at increased risk of hypoglycemia
Efficacy and safety evaluated in pediatric patients with T1D
Safety study in pregnant women with type 1 diabetes
As demonstrated in the BEGIN clinical trial program
Tresiba® offers proven A1C control1
ADULTS WITH T1D
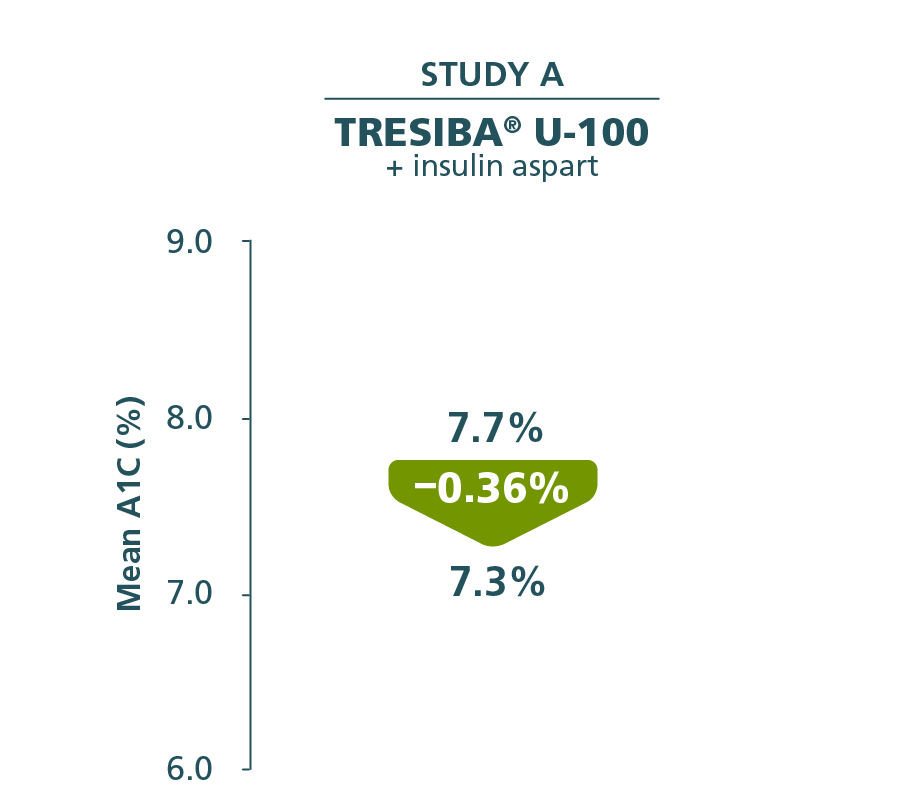
Comparator mean change from baseline
Insulin glargine U-100 + insulin aspart
Baseline: 7.7%
End of trial: 7.3%
Reduction: –0.34%
Primary endpoint in Study A:
A1C reduction from baseline through Week 52.2
- Difference between Tresiba® U-100 and insulin glargine U-100: –0.01% (95% CI, –0.14%; 0.11%)1
A1C results were similar between Tresiba® and insulin glargine in the other 6 clinical trials.
T1D=type 1 diabetes.
Secondary endpoint
FPG reductions with Tresiba®1
ADULTS WITH T1D

Comparator mean change from baseline
Insulin glargine U-100 + insulin aspart
Baseline: 174 mg/dL
End of trial: 149 mg/dL
Reduction: –21.6 mg/dL
Secondary endpoint in Study A:
FPG reduction from baseline through Week 52.2
FPG results were similar between Tresiba® and insulin glargine U-100 in the other 6 clinical trials.
FPG=fasting plasma glucose.
Hypoglycemia incidence rates were comparable among Tresiba® and basal insulin comparators1
Percentage of patients who experienced at least 1 episode of severe or Novo Nordisk–defined hypoglycemiaa,b
- Incidence rates in all T1D adult trials (range): 10.4% to 12.7% for severe hypoglycemiaa and 93.0% to 99.4% for Novo Nordisk–defined hypoglycemiab (basal-bolus regimen)
aSevere hypoglycemia: an event requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
bNovo Nordisk–defined hypoglycemia: a severe hypoglycemia event or an event where laboratory or self-measured glucose calibrated to plasma was <56 mg/dL or where whole blood glucose was <50 mg/dL (ie, with or without the presence of hypoglycemic symptoms).
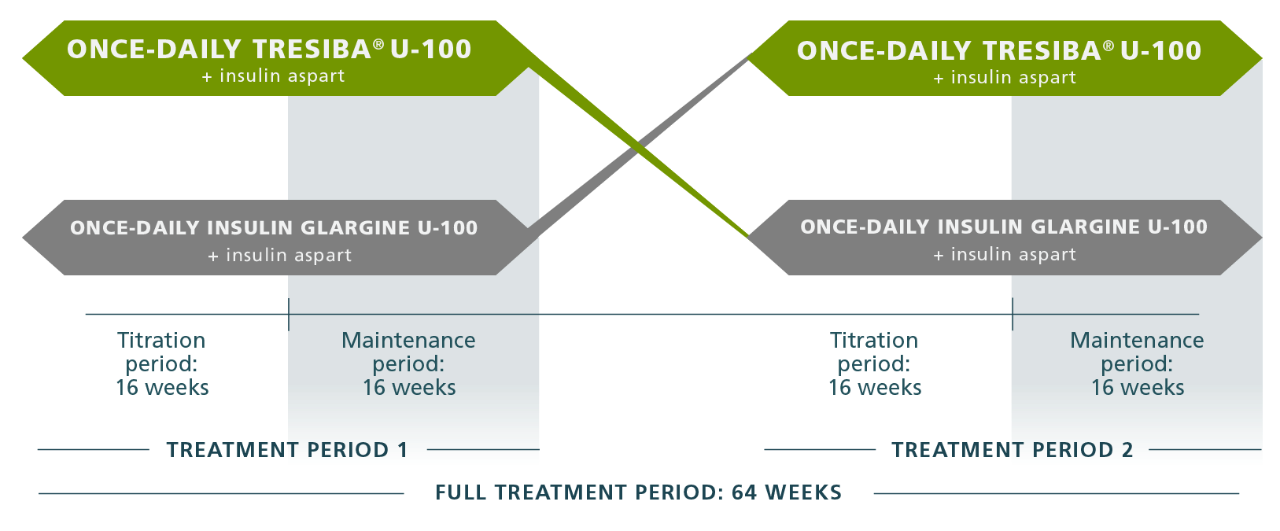
SWITCH 1 study design3
The SWITCH 1 trial was designed to further evaluate the safety profile of Tresiba® U-100 by comparing hypoglycemia rates of Tresiba® U-100 and insulin glargine U-1003
20% dose reduction
for basal and bolus insulin at randomization and at crossover3,c
501 adult patients
with T1D on continuous subcutaneous insulin infusion or once- or twice-daily basal insulin (NPH, insulin detemir, or insulin glargine U-100) with 2 to 4 bolus injections3
All patients were required to meet ≥1 of these criteria for hypoglycemia3:
- ≥1 episode of severe hypoglycemiad in previous year
- Moderate chronic renal failure (eGFR: 30–59 mL/min/1.73 m2)
- Hypoglycemic symptom unawareness
- Diabetes for >15 years
- Episode of hypoglycemiae within previous 12 weeks
Treatment arm 1

Treatment arm 2

Full treatment period: 64 weeks
A1C NONINFERIORITY IN BOTH TREATMENT PERIODS WAS A PREREQUISITE
Primary endpoint (noninferiority) was achieved, then tested for superiority: Tresiba® demonstrated superiority vs insulin glargine U-100 in terms of rates of severe or BG-confirmed symptomatic hypoglycemic episodes during the maintenance period.3
This study design ensured that all patients were exposed to each trial medication for the same amount of time.
Patient population was considered to be at increased risk for hypoglycemia.
cAlgorithm users only.
dBased on American Diabetes Association definition.
eSymptoms and/or BG level <70 mg/dL.
BG=blood glucose; eGFR=estimated glomerular filtration rate; NPH=neutral protamine Hagedorn.
SWITCH 1 trial: Designed to further evaluate the safety profile of Tresiba® in adult patients with T1D at increased risk of hypoglycemia
Lower rates of hypoglycemia with Tresiba® U-100 vs insulin glargine U-100 in patients with type 1 diabetes3
Primary endpoint
(maintenance period)
Severe or BG-confirmed symptomatic hypoglycemiaf
11%
LOWER RATE
(P<0.001)
Event ratesg:
insulin glargine U-100=2463,
Tresiba® U-100=2201
Confirmatory secondary endpoint
(maintenance period)
Nocturnal hypoglycemiaf
36%
LOWER RATE
(P<0.001)
Event ratesg:
insulin glargine U-100=429,
Tresiba® U-100=277
Supportive secondary endpoint
(maintenance period)
Severe hypoglycemiaf
35%
LOWER RATE
(P<0.001)
Event ratesg:
insulin glargine U-100=92,
Tresiba® U-100=69
- In the maintenance period, the percentage of patients experiencing at least 1 episode of severe hypoglycemia was: Tresiba® U-100, 10.3%; insulin glargine U-100, 17.1% (P=0.002)
- In the type 1 diabetes adult trials within the BEGIN clinical trial program, the percentage of patients experiencing at least 1 episode ranged from 10.4% to 12.7% for severe hypoglycemia and 93.0% to 99.4% for Novo Nordisk–defined hypoglycemia in trials with Tresiba® in a basal-bolus regimen.1,h,i
fBG-confirmed symptomatic hypoglycemia was defined as a BG measurement of <56 mg/dL with symptoms; severe hypoglycemia was defined per ADA 2013 guidelines.3
gAll event rates are episodes per 100 patient-years of exposure.
hSevere hypoglycemia: an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
iNovo Nordisk–defined hypoglycemia: a severe hypoglycemic event or an event where laboratory or self-measured glucose calibrated to plasma was <56 mg/dL or where whole BG was <50 mg/dL (ie, with or without the presence of hypoglycemic symptoms).1
ADA=American Diabetes Association; BG=blood glucose.
Achieved with similar glycemic control: Tresiba® vs insulin glargine U-100
In the maintenance period, the percentage of patients experiencing at least 1 episode of severe hypoglycemia was: Tresiba®, 10.3%; insulin glargine U-100, 17.1% (P=0.002).3
Secondary endpoints achieved3
- The rate of nocturnal symptomatic hypoglycemiai during the maintenance period was 36% lower with Tresiba® U-100 vs insulin glargine U-100 (277.1 episodes/100 PYE vs 428.6 episodes/100 PYE, respectively [95% CI, 0.56 to 0.73, relevant to rate ratio of 0.64]; P<0.001; rate difference, –61.94 episodes/100 PYE [95% CI, –83.85 to –40.03])3
- The rate of severe hypoglycemiaj was significantly lower during the maintenance period among those treated with insulin degludec than those treated with insulin glargine U-100 (69.1 episodes/100 PYE vs 92.2 episodes/100 PYE) for a rate ratio of 0.65 (95% CI, 0.48 to 0.89; P=0.007) and a rate difference of –13.65 (95% CI, –23.66 to –3.65). This trend continued during the full treatment period (86.8 episodes/100 PYE vs 105.2 episodes/100 PYE) for a rate ratio of 0.74 (95% CI, 0.61 to 0.90; P=0.003) and a rate difference of –6.84 (95% CI, –11.73 to –1.96)3
- The difference in proportions of patients experiencing 1 or more episodes of severe hypoglycemia during the maintenance period was not significantly different overall3
jNocturnal hypoglycemia was defined as any episode occurring between 12:01 AM and 5:59 AM, both inclusive; and severe hypoglycemia was defined per ADA 2013 guidelines.
PYE=patient-years of exposure; CI=confidence interval.
For your pediatric patients with T1D as young as 1 year of age
Tresiba® provides proven A1C reduction with once-daily dosing
In BEGIN YOUNG (Study J)
Changes in A1C from baseline at Week 26 (primary endpoint)1,4
PEDIATRIC PATIENTS WITH T1D
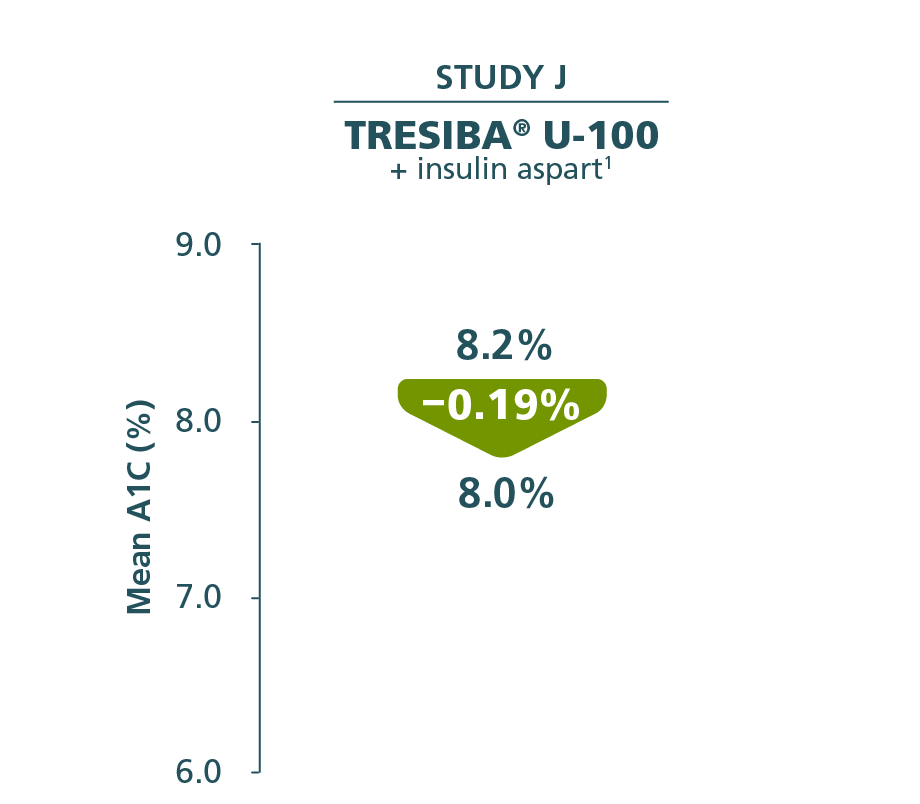
Comparator mean change from baseline
Insulin detemir + insulin aspart
Baseline: 8.0%
End of trial: 7.7%
Reduction: –0.34%

Secondary endpoint
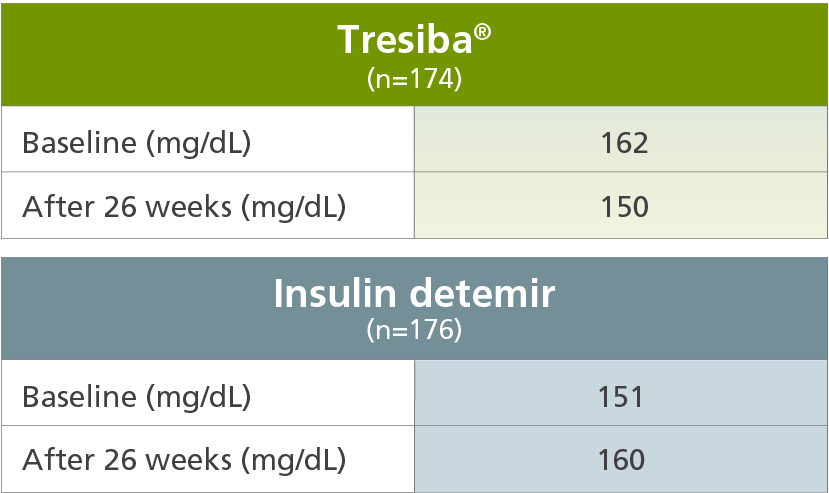
Change in FPG from baseline at Week 26 (secondary endpoint)1,4
Tresiba® compared with insulin detemir
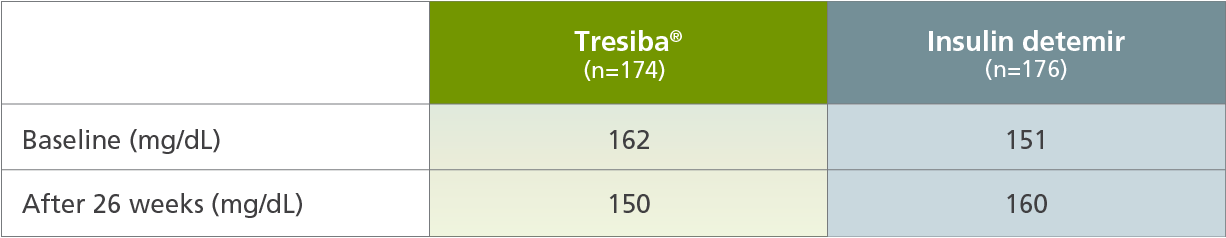
The mean age of the trial population was 10 years; 24% were ages 1 to 5 years, 39% were ages 6 to 11 years, and 36% were ages 12 to 17 years.
Tresiba® safety results in pediatric patients with T1D1,4
Tresiba® compared with insulin detemir
- The percentage of patients who experienced at least 1 episode of hypoglycemia was 17.8% for severe hypoglycemiak and 98.3% for Novo Nordisk–defined hypoglycemial
kSevere hypoglycemia in pediatric patients: an episode with altered mental status, where the child could not assist in his own care, was semiconscious or unconscious, or was in a coma ± convulsions and may require parenteral therapy (glucagon or intravenous glucose).
lNovo Nordisk–defined hypoglycemia: a severe hypoglycemia event or an event where laboratory or self-measured glucose calibrated to plasma was <56 mg/dL or where whole blood glucose was <50 mg/dL (ie, with or without the presence of hypoglycemic symptoms).
Ready to start a pediatric patient on Tresiba®?
Results from EXPECT clinical trial
Pregnancy outcomes in open-label trial of Tresiba® vs detemir1,5
An open-label, randomized trial of women aged ≥18 years with T1D and previously treated with insulin, who were at 8-13 weeks’ gestation or planned to become pregnant within 52 weeks (N=225). During the trial, 185 women (degludec: n=91; detemir: n=94) were pregnant with a singleton fetus.1,5
Pregnancy outcomes and the health of the fetus and newborn with Tresiba® resulted in no significant drug-associated differences vs insulin detemir in an open-label controlled clinical trial in type 1 diabetes.1
- In this study, the proportion of subjects with severe hypoglycemia and hypoglycemia was similiar between the 2 treatment arms1
- In about two-thirds of infants, insulin degludec was detected in the infant cord blood at levels above the lower level of quantification of the assay1
If left untreated, poorly controlled diabetes in pregnancy poses a risk to the mother and fetus.1
Study designs
Study A
BEGIN BASAL-BOLUS T1DM LONG1
Population: Adults with T1D.1
Patients randomized: Tresiba® (n=472); insulin glargine U-100 (n=157).1
Study design: 52-week, randomized, controlled, open-label, multinational, parallel-design, treat-to-target, noninferiority trial comparing the efficacy and safety of once-daily Tresiba® and once-daily insulin glargine U-100. Insulin aspart was administered before each meal in both treatment arms.
Basal insulin was titrated once weekly to an FPG target of 70 mg/dL to 90 mg/dL according to mean prebreakfast self-measured plasma glucose (SMPG) values (mean of 3 consecutive days). Bolus insulin was titrated to preprandial and bedtime SMPG concentrations of 70 mg/dL to 90 mg/dL.1,2
Primary endpoint: Change in A1C from baseline after 52 weeks of treatment.2
Secondary endpoints: FPG, SMPG, health-related quality of life, hypoglycemia, body weight, and lipids. Other safety endpoints were assessed in participants exposed to treatment.2
Mean end-of-trial basal and bolus insulin doses: Tresiba® arm: Tresiba®, 29 units; insulin aspart, 32 units; insulin glargine U-100 arm: insulin glargine U-100, 31 units; insulin aspart, 35 units.1
SWITCH 13
Population: Adult patients with T1D and who had ≥1 risk factor for hypoglycemia and who had been previously treated with either a basal-bolus regimen or CSII for 26 weeks or more. Patients also had to have A1C ≤10 and BMI of ≤45.
Study design: Randomized, double-blind, 2-period crossover, multicenter, treat-to-target trial comparing the rates of hypoglycemia associated with Tresiba® U-100 and insulin glargine U-100. Patients received Tresiba® U-100 once daily or insulin glargine U-100 once daily, both with insulin aspart, 2 to 4 times daily, for 2 consecutive 32-week periods and 1 week of follow-up. The starting dose of basal insulin and total bolus insulin (algorithm users only) was reduced by 20% at randomization and at crossover (ie, after 32 weeks). Patients were supplied with a blood glucose monitor and were instructed to measure their blood glucose level before breakfast, lunch, main evening meal, and bedtime on all days throughout the trial and whenever a hypoglycemic episode was suspected.
Primary endpoint: Rate of overall severem or blood glucose–confirmedn symptomatic hypoglycemic episodes during the maintenance period (Weeks 16 to 32 and 48 to 64).
Secondary endpoints: Rate of nocturnal symptomatic hypoglycemic episodes (severe or blood glucose–confirmed, occurring between 12:01 am and 5:59 am [both inclusive]) and the proportion of patients experiencing 1 or more severe hypoglycemic episodes, both in the maintenance period.3
mAn episode requiring third-party assistance, externally adjudicated.3
nPlasma glucose <56 mg/dL.3
CSII=continuous subcutaneous insulin infusion.
Study J
BEGIN YOUNG4
Population: Pediatric patients with T1D.4
Study design: 26-week, randomized, controlled, open-label, multinational, parallel-group, treat-to-target noninferiority trial comparing the efficacy and safety of once-daily Tresiba® U-100 (n=174) and once- or twice-daily insulin detemir (n=176). Subjects on a twice-daily insulin detemir regimen were dosed at breakfast and in the evening, either with the main evening meal or at bedtime. Insulin aspart was administered before each main meal in both treatment arms. Once-daily basal insulin was titrated to an FPG target of 90 mg/dL to 145 mg/dL according to lowest prebreakfast SMPG values (3 consecutive days). Bolus insulin was titrated to preprandial (of next meal) either by use of plasma glucose correction factors and insulin: carbohydrate ratios or once weekly, based on the lowest of 3 SMPG values measured 3 days before a visit/phone contact. Bolus insulin was also titrated to a 90 to 145 mg/dL target.1,4
Primary endpoint: Change in A1C from baseline after 26 weeks of treatment.1,4
Mean end-of-trial basal and bolus insulin doses: Tresiba® arm: Tresiba®, 16 units; insulin aspart, 23 units; insulin detemir arm: insulin detemir, 22 units; insulin aspart, 22 units.1
EXPECT Trial1,5
Population: Adult pregnant females with type 1 diabetes.
Patients randomized: Tresiba® (once daily) or insulin detemir (once or twice daily); both groups received insulin aspart 2 to 4 times daily with meals.
Study design: The EXPECT study is an open-label, randomized trial of 185 pregnant women aged ≥18 years with type 1 diabetes and who were previously treated with insulin and were at 8-13 weeks' gestation or planned to become pregnant within 52 weeks. Poor glucose control during pregnancy in both groups and small sample size were limitations of the study.
Primary endpoint: The primary analysis aimed to demonstrate the noninferiority (margin of 0.4%) of Tresiba® to insulin detemir with respect to the last planned glycated hemoglobin (HbA1c) measurement prior to delivery (>16 weeks’ gestation) using ANCOVA.
Secondary endpoints: Secondary endpoints were pregnancy outcomes as well as maternal efficacy and safety outcomes.
ANCOVA=analysis of covariance.
Important Safety Information for Tresiba®
Contraindications
- Tresiba® is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin degludec or any of the excipients in Tresiba®
Warnings and Precautions
- Never Share a Tresiba® FlexTouch® Pen, Needle, or Syringe Between Patients, even if the needle is changed. Patients using Tresiba® vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction of insulin, including Tresiba®. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) or who experience recurrent hypoglycemia. The long-acting effect of Tresiba® may delay recovery from hypoglycemia compared to shorter-acting insulins.
Risk Factors for Hypoglycemia: The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Tresiba® may vary among different patients or at different times in the same patients and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to concomitant drugs. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended. - Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products have been reported. To avoid medication errors between Tresiba® and other insulins, always instruct patients to always check the insulin label before each injection. To avoid dosing errors and potential overdose, never use a syringe to remove Tresiba® from the Tresiba® FlexTouch® disposable insulin prefilled pen.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Tresiba®. If hypersensitivity reactions occur, discontinue Tresiba®; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including Tresiba®, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Tresiba®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions commonly associated with Tresiba® are hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Drug Interactions
- There are certain drugs that may cause clinically significant drug interactions with Tresiba®.
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine
Please click here for Tresiba® Prescribing Information.
Important Safety Information for Tresiba®
Contraindications
- Tresiba® is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin degludec or any of the excipients in Tresiba®
Warnings and Precautions
- Never Share a Tresiba® FlexTouch® Pen, Needle, or Syringe Between Patients, even if the needle is changed. Patients using Tresiba® vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction of insulin, including Tresiba®. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) or who experience recurrent hypoglycemia. The long-acting effect of Tresiba® may delay recovery from hypoglycemia compared to shorter-acting insulins.
Risk Factors for Hypoglycemia: The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Tresiba® may vary among different patients or at different times in the same patients and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to concomitant drugs. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended. - Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products have been reported. To avoid medication errors between Tresiba® and other insulins, always instruct patients to always check the insulin label before each injection. To avoid dosing errors and potential overdose, never use a syringe to remove Tresiba® from the Tresiba® FlexTouch® disposable insulin prefilled pen.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Tresiba®. If hypersensitivity reactions occur, discontinue Tresiba®; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including Tresiba®, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Tresiba®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions commonly associated with Tresiba® are hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Drug Interactions
- There are certain drugs that may cause clinically significant drug interactions with Tresiba®.
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine
Please click here for Tresiba® Prescribing Information.
References:
- Tresiba [package insert]. Plainsboro, NJ: Novo Nordisk Inc; July 2022.
- Heller S, Buse J, Fisher M, et al. Lancet. 2012;379(9825):1489-1497.
- Lane W, Bailey TS, Gerety G, et al. JAMA. 2017;318(1):33-44.
- Thalange N, Deeb L, Iotova V, et al. Pediatr Diabetes. 2015;16(3):164-176.
- Data on file. Novo Nordisk Inc; July 2021.