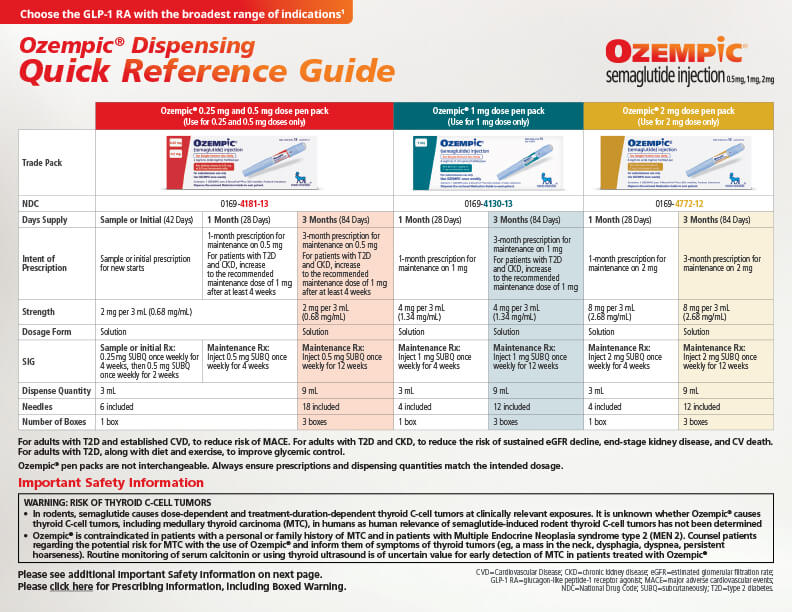
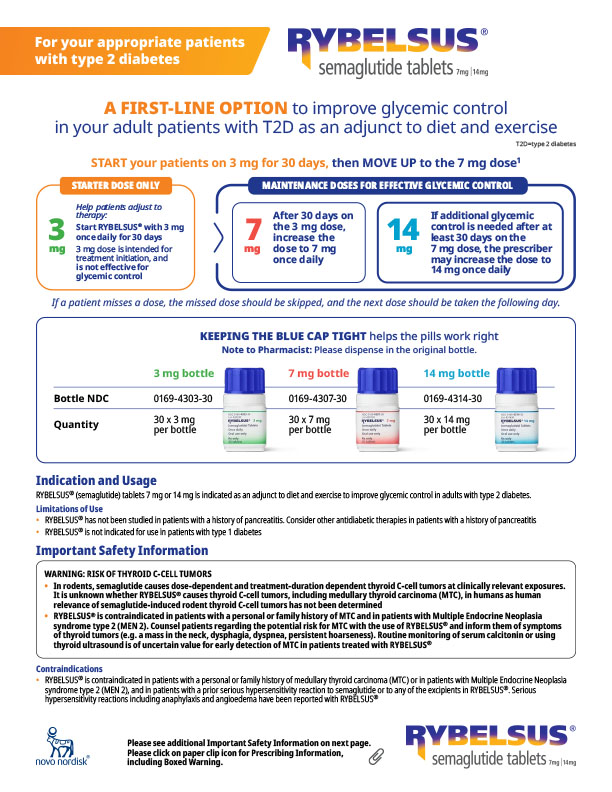
Product resources library
Explore our library of diabetes treatment education, including clinical trial data, pharmacokinetics, mechanisms of action, and treatment options to support individualized patient care.
For adults with type 2 diabetes as an adjunct to diet and exercise to improve glycemic control. To reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk of these events.
For adults with type 2 diabetes (T2D), along with diet and exercise to improve glycemic control, for adults with T2D and established CVD, to reduce risk of MACE (CV death, nonfatal MI, or nonfatal stroke), and for adults with T2D and chronic kidney disease, to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death.
A basal insulin to improve glycemic control in patients one year of age or older with diabetes.
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Contraindications
- RYBELSUS® is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with RYBELSUS®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS®. Observe patients carefully for signs and symptoms of acute pancreatitis which may include persistent or severe abdominal pain (sometimes radiating to the back), nausea or vomiting. If pancreatitis is suspected, discontinue RYBELSUS® and initiate appropriate management
- Diabetic Retinopathy Complications: In a pooled analysis of glycemic control trials with RYBELSUS®, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS® and 3.8% with comparator). In a 2-year trial with semaglutide injection involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with semaglutide injection (3.0%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Hypoglycemia: Patients receiving RYBELSUS® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who had experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to RYBELSUS® that could lead to volume depletion, especially during initiation and escalation of RYBELSUS®
- Severe Gastrointestinal Adverse Reactions: Use of RYBELSUS® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving RYBELSUS® (7 mg 0.6%, 14 mg 2%) than placebo (0.3%). RYBELSUS® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with RYBELSUS®. If hypersensitivity reactions occur, discontinue use of RYBELSUS®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS® 7 mg. In a 4-year cardiovascular outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS® 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% treated with RYBELSUS® 14 mg and in 0.7% of placebo-treated patients. If cholelithiasis or cholecystitis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: RYBELSUS® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking RYBELSUS®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are nausea, abdominal pain, diarrhea, decreased appetite, vomiting and constipation
Drug Interactions
- RYBELSUS® stimulates insulin release in the presence of elevated blood glucose concentrations. When initiating RYBELSUS®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- RYBELSUS® delays gastric emptying and has the potential to impact the absorption of other oral medications. Closely follow RYBELSUS® administration instructions when coadministering with other oral medications and consider increased monitoring for medications with a narrow therapeutic index, such as levothyroxine
Use in Specific Populations
- Pregnancy: Available data with RYBELSUS® are not sufficient to determine a drug associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Discontinue RYBELSUS® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for RYBELSUS®, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with RYBELSUS®
Please click here for RYBELSUS® Prescribing Information, including Boxed Warning.
Indication and Usage
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk for these events
Important Safety Information for Tresiba®
Contraindications
- Tresiba® is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin degludec or any of the excipients in Tresiba®
Warnings and Precautions
- Never Share a Tresiba® FlexTouch® Pen, Needle, or Syringe Between Patients, even if the needle is changed. Patients using Tresiba® vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction of insulin, including Tresiba®. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) or who experience recurrent hypoglycemia. The long-acting effect of Tresiba® may delay recovery from hypoglycemia compared to shorter-acting insulins.
Risk Factors for Hypoglycemia: The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Tresiba® may vary among different patients or at different times in the same patients and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to concomitant drugs. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended. - Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products have been reported. To avoid medication errors between Tresiba® and other insulins, always instruct patients to always check the insulin label before each injection. To avoid dosing errors and potential overdose, never use a syringe to remove Tresiba® from the Tresiba® FlexTouch® disposable insulin prefilled pen.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Tresiba®. If hypersensitivity reactions occur, discontinue Tresiba®; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including Tresiba®, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Tresiba®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions commonly associated with Tresiba® are hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Drug Interactions
- There are certain drugs that may cause clinically significant drug interactions with Tresiba®.
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine
Please click here for Tresiba® Prescribing Information.
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Contraindications
- RYBELSUS® is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with RYBELSUS®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS®. Observe patients carefully for signs and symptoms of acute pancreatitis which may include persistent or severe abdominal pain (sometimes radiating to the back), nausea or vomiting. If pancreatitis is suspected, discontinue RYBELSUS® and initiate appropriate management
- Diabetic Retinopathy Complications: In a pooled analysis of glycemic control trials with RYBELSUS®, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS® and 3.8% with comparator). In a 2-year trial with semaglutide injection involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with semaglutide injection (3.0%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Hypoglycemia: Patients receiving RYBELSUS® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who had experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to RYBELSUS® that could lead to volume depletion, especially during initiation and escalation of RYBELSUS®
- Severe Gastrointestinal Adverse Reactions: Use of RYBELSUS® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving RYBELSUS® (7 mg 0.6%, 14 mg 2%) than placebo (0.3%). RYBELSUS® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with RYBELSUS®. If hypersensitivity reactions occur, discontinue use of RYBELSUS®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS® 7 mg. In a 4-year cardiovascular outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS® 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% treated with RYBELSUS® 14 mg and in 0.7% of placebo-treated patients. If cholelithiasis or cholecystitis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: RYBELSUS® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking RYBELSUS®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are nausea, abdominal pain, diarrhea, decreased appetite, vomiting and constipation
Drug Interactions
- RYBELSUS® stimulates insulin release in the presence of elevated blood glucose concentrations. When initiating RYBELSUS®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- RYBELSUS® delays gastric emptying and has the potential to impact the absorption of other oral medications. Closely follow RYBELSUS® administration instructions when coadministering with other oral medications and consider increased monitoring for medications with a narrow therapeutic index, such as levothyroxine
Use in Specific Populations
- Pregnancy: Available data with RYBELSUS® are not sufficient to determine a drug associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Discontinue RYBELSUS® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for RYBELSUS®, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with RYBELSUS®
Please click here for RYBELSUS® Prescribing Information, including Boxed Warning.
Indication and Usage
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk for these events
Important Safety Information for Tresiba®
Contraindications
- Tresiba® is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin degludec or any of the excipients in Tresiba®
Warnings and Precautions
- Never Share a Tresiba® FlexTouch® Pen, Needle, or Syringe Between Patients, even if the needle is changed. Patients using Tresiba® vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction of insulin, including Tresiba®. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) or who experience recurrent hypoglycemia. The long-acting effect of Tresiba® may delay recovery from hypoglycemia compared to shorter-acting insulins.
Risk Factors for Hypoglycemia: The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Tresiba® may vary among different patients or at different times in the same patients and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to concomitant drugs. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended. - Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products have been reported. To avoid medication errors between Tresiba® and other insulins, always instruct patients to always check the insulin label before each injection. To avoid dosing errors and potential overdose, never use a syringe to remove Tresiba® from the Tresiba® FlexTouch® disposable insulin prefilled pen.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Tresiba®. If hypersensitivity reactions occur, discontinue Tresiba®; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including Tresiba®, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Tresiba®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions commonly associated with Tresiba® are hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Drug Interactions
- There are certain drugs that may cause clinically significant drug interactions with Tresiba®.
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine
Please click here for Tresiba® Prescribing Information.