The Power of Pharmacists: Your role with RYBELSUS® (semaglutide)
Resources that help you support patients as they start treatment and continue through dose escalation.
RYBELSUS® is the only FDA-approved semaglutide in a pill for adults with T2D.1 Here is some important information to be sure to discuss with your patients when they come to the pharmacy.
Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
T2D=type 2 diabetes.
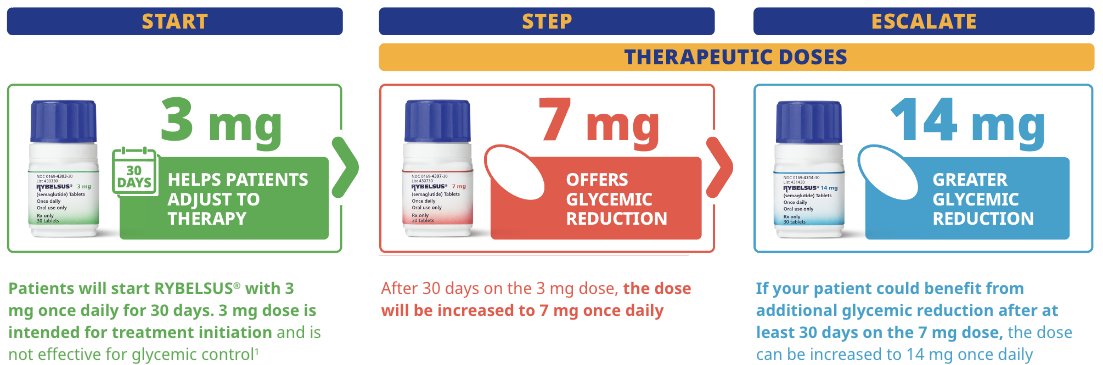
Dosing and administration
Savings and
support
How to dispense
Get updates
Dosing and administration
Savings and support
How to dispense
Get updates
Storing RYBELSUS®1
Store tablets in the original closed RYBELSUS® bottle until ready to take one
Do not use a pill organizer or any other container to store RYBELSUS® tablets
Important note: The RYBELSUS® blue cap is equipped with a drying agent to help protect the tablets from moisture, which helps preserve RYBELSUS®. Tablets should be kept in the original RYBELSUS® bottle with the blue cap whenever the patient is not taking one.
Store at room temperature 68 °F–77 °F (20 °C–25 °C). Store in a dry place away from moisture.
Taking RYBELSUS®1
Take RYBELSUS® in the morning
Patients must take RYBELSUS® on an empty stomach.
Take with no more than 4 oz of water
Do not take RYBELSUS® with any other liquids besides water.
Wait 30 minutes before the first food, beverage, or other oral medications of the day
Waiting less than 30 minutes or taking with food, beverages (other than plain water), or other oral medications will lessen the effect of RYBELSUS® by decreasing absorption. Waiting more than 30 minutes to eat may increase the absorption of RYBELSUS®.
Swallow tablet whole
Do not split, crush, or chew
Do not take more than 1 tablet per day.
Understanding common side effects1
The most common adverse reactions, reported in ≥5% of patients treated with RYBELSUS® are:
- nausea
- abdominal pain
- diarrhea
- decreased appetite
- vomiting
- constipation
In the pool of placebo-controlled trials, the majority of reports of nausea, vomiting, and/or diarrhea occurred during dose escalation.
Tips for nausea2
Here are some general nausea tips that may be helpful for patients:
Eat slowly and eat smaller, more frequent meals
Eat foods that are light and bland, like saltine crackers or plain bread
Avoid fried, greasy, or sweet foods
Drink clear or ice-cold drinks
Text READY to 21848a
Patients can receive copay savings and text messages to help them start and stay on RYBELSUS®
Call 1-833-ASK-A-CDE
Patients can ask for assistance with the savings offer Monday–Friday, 9:00 AM–6:00 PM ET
9 out of 10 Commercial and Medicare Part D patients are covered for RYBELSUS®
FOR YOUR COMMERCIALLY INSURED PATIENTS
~98% Commercial coverage nationally1,b

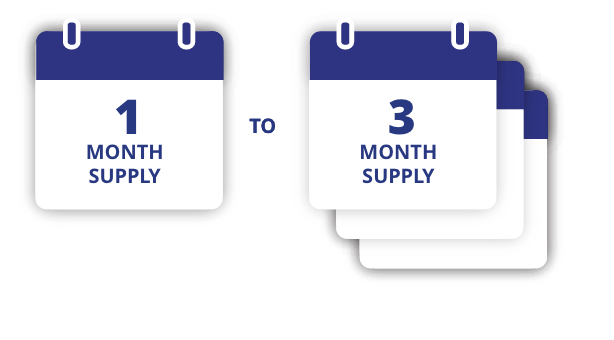
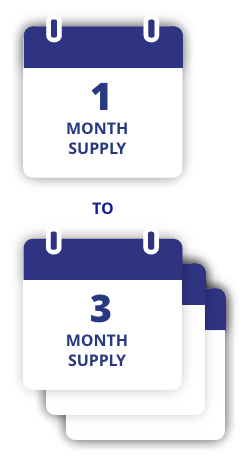
To receive offer, 7 mg or 14 mg dose prescription must be for a 1-, 2-, or 3-month supply. For 3 mg dose, offer is limited to a 1-month supply only.
FOR YOUR MEDICARE PART D INSURED PATIENTS
~95% Part D coverage nationally1,b
1 in 2 patients with type 2 diabetes on Medicare may qualify for full Extra Help (LIS), and pay no more than $12.15* for RYBELSUS® per month3
*Centers for Medicare and Medicaid Services, Department of Health & Human Services. Medicare & You 2023: The official U.S. government Medicare handbook. https://www.medicare.gov/publications/10050-Medicare-and-You.pdf. Accessed January 3, 2023.
aMessage and data rates may apply. Check with your mobile service provider. See Terms & Conditions of Use at RYBELSUS.com.
bNovo Nordisk defines access as covered when brand is available on formulary with or without restrictions. Coverage status and tier vary by plan and are subject to change. Please check directly with the health plan to confirm coverage for individual patients.
cOffer available only to commercially insured patients with RYBELSUS® coverage. Month is defined as 30 days. Maximum savings of $300 per 1-month supply, $600 per 2-month supply, or $900 per 3-month supply. Eligibility and other restrictions apply.
Ordering RYBELSUS®
Information you need when ordering RYBELSUS® for your pharmacy.
STARTER DOSE

3 mg
STRENGTH
3 mgd
NDC NUMBER
0169-4303-30
BOTTLE QUANTITY
30 x 3 mg per bottle
THERAPEUTIC DOSE

7 mg
STRENGTH
7 mg
NDC NUMBER
0169-4307-30
BOTTLE QUANTITY
30 x 7 mg per bottle
THERAPEUTIC DOSE

14 mg
STRENGTH
14 mg
NDC NUMBER
0169-4314-30
BOTTLE QUANTITY
30 x 14 mg per bottle
dThe 3 mg dose is intended for treatment initiation and is not effective for glycemic control.
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Contraindications
- RYBELSUS® is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with RYBELSUS®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS®. Observe patients carefully for signs and symptoms of acute pancreatitis which may include persistent or severe abdominal pain (sometimes radiating to the back), nausea or vomiting. If pancreatitis is suspected, discontinue RYBELSUS® and initiate appropriate management
- Diabetic Retinopathy Complications: In a pooled analysis of glycemic control trials with RYBELSUS®, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS® and 3.8% with comparator). In a 2-year trial with semaglutide injection involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with semaglutide injection (3.0%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Hypoglycemia: Patients receiving RYBELSUS® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who had experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to RYBELSUS® that could lead to volume depletion, especially during initiation and escalation of RYBELSUS®
- Severe Gastrointestinal Adverse Reactions: Use of RYBELSUS® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving RYBELSUS® (7 mg 0.6%, 14 mg 2%) than placebo (0.3%). RYBELSUS® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with RYBELSUS®. If hypersensitivity reactions occur, discontinue use of RYBELSUS®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS® 7 mg. In a 4-year cardiovascular outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS® 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% treated with RYBELSUS® 14 mg and in 0.7% of placebo-treated patients. If cholelithiasis or cholecystitis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: RYBELSUS® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking RYBELSUS®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are nausea, abdominal pain, diarrhea, decreased appetite, vomiting and constipation
Drug Interactions
- RYBELSUS® stimulates insulin release in the presence of elevated blood glucose concentrations. When initiating RYBELSUS®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- RYBELSUS® delays gastric emptying and has the potential to impact the absorption of other oral medications. Closely follow RYBELSUS® administration instructions when coadministering with other oral medications and consider increased monitoring for medications with a narrow therapeutic index, such as levothyroxine
Use in Specific Populations
- Pregnancy: Available data with RYBELSUS® are not sufficient to determine a drug associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Discontinue RYBELSUS® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for RYBELSUS®, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with RYBELSUS®
Please click here for RYBELSUS® Prescribing Information, including Boxed Warning.
Indication and Usage
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk for these events
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Important Safety Information for RYBELSUS®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether RYBELSUS® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- RYBELSUS® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of RYBELSUS® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with RYBELSUS®
Contraindications
- RYBELSUS® is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in RYBELSUS®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with RYBELSUS®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including RYBELSUS®. Observe patients carefully for signs and symptoms of acute pancreatitis which may include persistent or severe abdominal pain (sometimes radiating to the back), nausea or vomiting. If pancreatitis is suspected, discontinue RYBELSUS® and initiate appropriate management
- Diabetic Retinopathy Complications: In a pooled analysis of glycemic control trials with RYBELSUS®, patients reported diabetic retinopathy related adverse reactions during the trial (4.2% with RYBELSUS® and 3.8% with comparator). In a 2-year trial with semaglutide injection involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with semaglutide injection (3.0%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Hypoglycemia: Patients receiving RYBELSUS® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who had experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to RYBELSUS® that could lead to volume depletion, especially during initiation and escalation of RYBELSUS®
- Severe Gastrointestinal Adverse Reactions: Use of RYBELSUS® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving RYBELSUS® (7 mg 0.6%, 14 mg 2%) than placebo (0.3%). RYBELSUS® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with RYBELSUS®. If hypersensitivity reactions occur, discontinue use of RYBELSUS®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials to improve glycemic control, cholelithiasis was reported in 1% of patients treated with RYBELSUS® 7 mg. In a 4-year cardiovascular outcomes trial (Trial 7), cholelithiasis was reported in 1.1% of patients treated with RYBELSUS® 14 mg and in 0.9% of placebo-treated patients. In Trial 7, cholecystitis was reported in 1.1% treated with RYBELSUS® 14 mg and in 0.7% of placebo-treated patients. If cholelithiasis or cholecystitis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: RYBELSUS® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking RYBELSUS®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are nausea, abdominal pain, diarrhea, decreased appetite, vomiting and constipation
Drug Interactions
- RYBELSUS® stimulates insulin release in the presence of elevated blood glucose concentrations. When initiating RYBELSUS®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- RYBELSUS® delays gastric emptying and has the potential to impact the absorption of other oral medications. Closely follow RYBELSUS® administration instructions when coadministering with other oral medications and consider increased monitoring for medications with a narrow therapeutic index, such as levothyroxine
Use in Specific Populations
- Pregnancy: Available data with RYBELSUS® are not sufficient to determine a drug associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Discontinue RYBELSUS® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for RYBELSUS®, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with RYBELSUS®
Please click here for RYBELSUS® Prescribing Information, including Boxed Warning.
Indication and Usage
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus who are at high risk for these events
References:
- RYBELSUS® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Nausea & vomiting. Cleveland Clinic. Updated August 2023. Accessed April 11, 2025. https://my.clevelandclinic.org/health/symptoms/8106-nausea--vomiting
- IQVIA LAAD 2022, Medicare patients.