Indicated for treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital factor VII (FVII) deficiency, Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, and in adults with acquired hemophilia.
Actor portrayal
"I'll never forget my first patient. When I saw a prolonged aPTT, I knew where to turn for a fast treatment."2
"I'll never forget my first patient. When I saw a prolonged aPTT, I knew where to turn for a fast treatment."2

Congenital hemophilia with inhibitors, as seen in the adept 2 clinical trial.2,3

Acquired hemophilia4
(first-line treatment)

Congenital Factor VII
deficiency2,5

Glanzmann’s
thrombasthenia2
Explore interactive dosing tools
See recommendations and use the calculator tool for perioperative and breakthrough bleed dosing across 5 approved indications.2
NovoSeven® RT for your hospital:
the broadest range of protectionb
Proven effective for bleed resolution and surgery across 5 indications.2
CHAwI
CHBwI
Acquired
hemophilia
GT
CFVIId
NovoSeven®RT2
(coagulation Factor VIIa, recombinant)





SEVENFACT®6
[coagulation factor VIIa (recombinant)-jncw]



FEIBA®7
(anti-inhibitor coagulant complex or pd-aPCC)





Obizur®8
[antihemophilic factor (recombinant), porcine sequence]




= bleed treatment;
= perioperative management
= routine prophylaxis
= pediatric indication

Not intended to be a comparison of efficacy or safety.
bIndicated for bleed control and surgery in 5 bleeding disorders.2
cFor patients aged 12 years and older.6
dObizur® is contraindicated in patients with congenital hemophilia A with inhibitors.8
CHAwI=congenital hemophilia A with inhibitors;
CHBwI=congenital hemophilia B with inhibitors;
CFVIId=congenital factor VII defi ciency; GT=Glanzmann’s Thrombasthenia with refractoriness to platelets; pd-aPCC=plasma-derived activated prothrombin complex concentrate
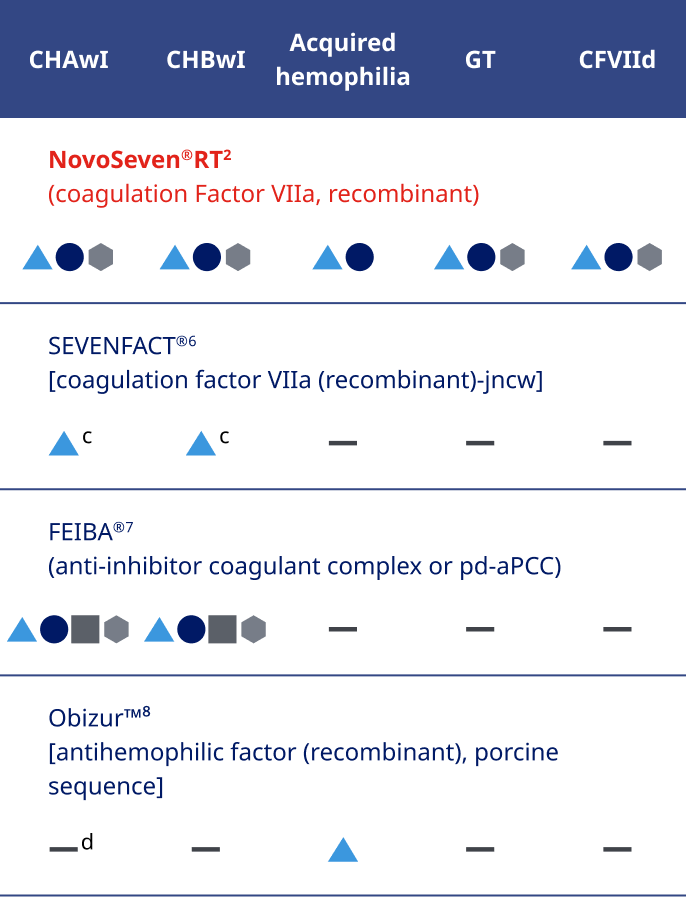
NovoSeven® RT offers
features to support hospital use2
Appropriate for a variety of hospital uses:
- Faster to infuse compared with FEIBA® and Obizur®2,6,7,e
- NovoSeven® RT has one dosing regimen for patients with CHwI regardless of bleed severity - That consistent dosing may lead to predictable utilization2
- Ability to administer via continuous infusion for perioperative management
- Recombinant safety and low rate of thrombotic adverse events based on clinical trials and registry data2,f
- The most common and serious adverse reaction in clinical trials are thrombotic events2,f
Efficient for your hospital pharmacy:
- Low-volume dosing and compact packaging to help maximize space
- Room temperature storage up to 77˚Fg
- Each vial comes with a prefilled syringe, meaning no extra steps to fill a syringe with diluenth
- Available in 1-mg, 2-mg, 5-mg, and 8-mg vials with everything needed for reconstitution is in 1 box
eAdminister NovoSeven® RT as a slow bolus injection over 2 to 5 minutes, depending on the dose administered.1 FEIBA should be injected or infused intravenously at a rate that does not exceed 10 units per kg of body weight per minute. For a dose of 75 U/kg, this equates to an infusion time of 7.5 minutes. Administer Obizur® intravenously at a rate of 1 to 2 mL per minute. For a 75 kg patient receiving a dose of 200 u/kg, this corresponds to an infusion time of 15 to 30 minutes.2,7,8
f0.2% in CHwI bleeds, 4% of patients with acquired hemophilia, <0.2% in GT bleeds, and 0.8% in patients with CFVIId bleeds.2
gPrior to reconstitution, store NovoSeven® RT powder and histidine diluent between 36-77˚F. After reconstitution, store NovoSeven® RT either at room temperature or refrigerated for up to 3 hours. Do not freeze reconstituted NovoSeven® RT or store in syringes.2
hCompared with reconstitution using histidine vials.
In the ER: be prepared for acute & emergent bleeding with NovoSeven® RT

- In situations where a patient does not bring their own clotting factor concentrate, MASAC recommends emergency departments have ready access to factor replacement product within 1 hour upon a patient’s arrival.8
- MASAC also recommends rFVIIa as a first option to treat acute bleeding episodes in patients with CHAwI on emicizumab—FEIBA® (pd-aPCC) should be avoided if possible.9
- For patients with CHAwI on emicizumab, duration of FEIBA® therapy should be minimized. Prolonged usage of FEIBA® for >24 hours with doses above 100 units/kg/day may be associated with thrombosis and TMA.9
Explore clinical pathways for
CHwI & acquired hemophilia
Is your hospital ready to treat emergent bleeds for CHwI patients?
A severe joint bleed doesn’t just mean danger today—delayed treatment of emergent joint bleeds in the CHwI population may result in cumulative damage.13
Take steps to see if your emergency department is prepared to stop bleeds & their consequences.
Explore the emergent bleeds clinical pathway
Actor Portrayal
Early diagnosis is crucial for treating acquired hemophilia—do you know the signs?
Acquired hemophilia (AH) is a rare and often idiopathic autoimmune condition that can cause severe and even fatal bleeding in a variety of patients.14
It's time to equip every department with resources and information to rapidly diagnose and treat AH.
Explore the AH clinical pathway
Actor Portrayal
Study design
Lentz, et al
adept™2 phase 3 trial
Patients randomized: Patients ≥12 years of age with hemophilia A with inhibitors (N=66) or hemophilia B with inhibitors (N=6) who have experienced at least 5 bleeds during treatment prior to entering the trial.
Study design: A 12-month, international, multicenter, randomized, double-blind, active-controlled, crossover, confirmatory phase 3 trial. Primarily done in the home setting, treatment of bleeding episodes were randomized, either treated with 1 to 3 doses of vatreptacog alfa (340 bleeding episodes) at 80 mcg/kg or 1 to 3 doses of NovoSeven® RT (227 bleeding episodes) at 90 mcg/kg.
Primary endpoint: Effective bleed control, which was defined as no additional treatment needed (other than the original medication) within 12 hours after first dose.
Secondary endpoint: Effective and sustained bleed control at 1 and 2 days after initial dose, number of doses of trial product for each bleed, and changes in pain assessment.
Sumner, et al
Compassionate use programs, the Hemostasis and Thrombosis Research Society (HTRS) registry, and independent published reports
Patients considered: Patients diagnosed with acquired hemophilia from compassionate and emergency use programs (N=61), the HTRS (N=9) and independent published reports (N=69).
Study design: Data were extracted from a review of experiences with recombinant FVIIa for the treatment of acquired hemophilia in compassionate and emergency use programs, the Hemostasis and Thrombosis Research Society (HTRS) registry, and independent published reports. Efficacy was evaluated similarly but not identically between compassionate use programs, the HTRS registry, and published reports.
Efficacy of NovoSeven® RT is based on pooled data from the NovoSeven® RT compassionate and emergency use programs (1988–1999), the Hemostasis and Thrombosis Research Society (HTRS) registry, and independent published reports from January 1999 to September 2005. The analysis includes 182 bleeding episodes in 139 patients; NovoSeven® RT was used as first-line and salvage therapy in 103 and 73 episodes, respectively. The percentage of effectiveness noted includes episodes that were evaluated as effective or partially effective.
Published literature, compassionate use trials, and the Hemophilia and Thrombosis Research Society (HTRS) Registry
Patients considered: Data was collected from published literature, compassionate use trials, and the HTRS for patients with congenital factor VII deficiency (N=70) treated with NovoSeven® RT.
Study design: NovoSeven® RT was used as treatment in 124 bleeding episodes, surgeries, or prophylaxis regimens. Dosing ranged from 6 to 98 mcg/kg administered every 2 to 12 hours (except for prophylaxis [doses administered from 2 times per day up to 2 times per week]). Patients were treated with an average of 1 to 10 doses. Treatment was effective if bleeding stopped or the physician rated the treatment as effective.
Glanzmann’s Thrombasthenia Registry (GTR)
Patients considered: Data was collected from the GTR in patients with Glanzmann’s thrombasthenia (N=218).
Study design: Adjudicator-assessed effectiveness of treatment regimens in patients with GT (N=218) in all severe bleeding episodes and all surgical procedures (N=1073) based on review of Glanzmann’s Thrombasthenia Registry (GTR) data unblinded to investigator-coded efficacy. Efficacy was evaluated on a 2-point scale (clinical assessment of success or failure of treatment regimen as a whole, blinded and unblinded to investigator-coded outcome) including 92 patients treated with NovoSeven® RT for 266 bleeding episodes and 77 patients treated for 160 surgical procedures.
Need administration information?
Looking for more professional education?
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
References
- Data on file as of 2023. Novo Nordisk Inc; Plainsboro, NJ.
- NovoSeven RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Lentz SR, Ehrenforth S, Abdul Karim F, et al; adept™2 investigators. Recombinant factor VIIa analog in the management of hemophilia with inhibitors: results from a multicenter, randomized, controlled trial of vatreptacog alfa. J Thromb Haemost. 2014;12(8):1244-1253.
- Sumner MJ, Geldziler BD, Pedersen M, Seremetis S. Treatment of acquired haemophilia with recombinant activated FVII: a critical appraisal. Haemophilia. 2007;13(5):451-461.
- Mariani G, Napolitano M, Dolce A, et al. Replacement therapy for bleeding episodes in factor VII deficiency. A prospective evaluation. Thromb Haemost. 2013;109:238–247.
- FEIBA [package insert]. Westlake Village, CA: Baxalta US Inc; 2024.
- Obizur [package insert]. Westlake Village, CA: Baxalta US Inc; 2024.
- National Hemophilia Foundation. MASAC Guidelines for Emergency Department Management of Individuals with Hemophilia and Other Bleeding Disorders, #257. 2019.
- National Hemophilia Foundation. Recommendation on the Use and Management of Emicizumab-kxwh (Hemlibra®) for Hemophilia A With and Without Inhibitors, #268. New York, NY: National Hemophilia Foundation; 2022.
- Levy GG, Asikanius E, Kuebler P, et al. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: experience from the HAVEN clinical program. J Thromb Haemost. 2019;17(9):1470-1477.
- Shapiro AD, Gilchrist GS, Hoots WK, et al. Prospective, randomised trial of two doses of rFVIIa (NovoSeven) in haemophilia patients with inhibitors undergoing surgery. Thromb Haemost. 1998;80(5):773-778.
- Lusher JM, Roberts HR, Davignon G, et al; and rFVIIa Study Group. A randomized, double-blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. Haemophilia. 1998;4(6):790-798.
- Salek SZ, Benson GM, Elezovic I, et al. The need for speed in in the management of haemophilia patients with inhibitors. Haemophilia. 2011;17(1):95-102.
- Huth-Kühne A, Baudo F, Collins P, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2020;94(4):566-575.
