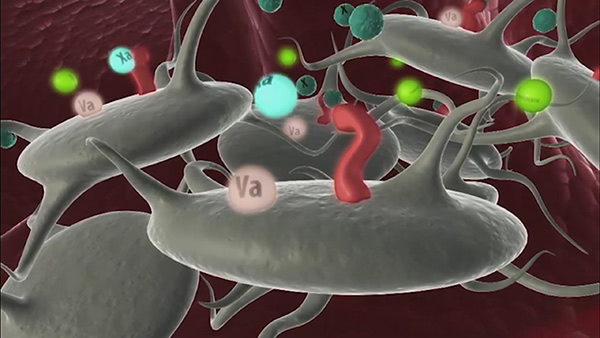
Indicated for treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital factor VII (FVII) deficiency, Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, and in adults with acquired hemophilia.
Education for you
Novo Nordisk provides professional educational materials to download or share with colleagues.
Education for you
Novo Nordisk provides professional educational materials to download or share with colleagues.
Actor portrayal
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia