Indicated for treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital factor VII (FVII) deficiency, Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, and in adults with acquired hemophilia.
“There’s one patient I’ll never forget. When it was time to consider a joint replacement surgery, we knew where to turn for effective treatment.“1
Actor portrayal
“There’s one patient I’ll never forget. When it was time to consider a joint replacement surgery, we knew where to turn for effective treatment.“1
Actor portrayal
The experience continues
NovoSeven® RT has 35+ years of research and long-term clinical experience; 1,106 patients and 5,305 bleeds treated in registrational studies and ~400 successful surgeries and procedures.1-25,a-c
Effective control of the mildest to most life-threatening bleeds
Proven effective for bleed resolution and surgery across 5 indications.1 NovoSeven® RT is the only rFVIIa indicated for CHwI patients of all ages.1
Clinical experience with Hemlibra®
NovoSeven® RT was the only recombinant factor used for breakthrough bleeds in patients with inhibitors in the Hemlibra® pivotal clinical trials.26,d
MASAC recommends rFVIIa to treat acute bleeds in patients with congenital hemophilia A with inhibitors taking emicizumab prophylaxis.27
Able to quickly treat bleeds when they occur
Rapid administration and infusion, leading to rapid activity.1,28 A median of 2 doses helped control joint bleeds in as little as 5 hours in CHwI patients.29,e
Portability to fit into your patients’ lives
Room temperature stable for rapid access to treatment. Everything patients need to reconstitute is in one small box.1,f
#1 prescribed rFVIIa in hospitals30,g
NovoSeven® RT is the only rFVIIa approved for surgery and for use in patients of all ages.1
A well-established safety profile
A low rate of thrombotic events based on clinical trials and registries.1,h
- 0.2% of bleeding episodes in patients with CHwI
- 4% in patients with AH
- <0.2% of bleeding episodes in patients with GT
- 0.8% of bleeding episodes in patients with CFVIId31,h
- The most common and serious adverse reactions in clinical trials are thrombotic events
And NovoSeven® RT is not made from human serum or human proteins.1,30
a1988: compassionate use initiated in the United States; 1999: FDA approval received for CHwI.30
bIncludes bleeding episodes, major and minor surgical procedures, traumatic injuries, and prophylaxis regimens.
cSuccess was defined differently in each study.
dThe analysis included bleeding episodes in the HAVEN1, HAVEN2, and HAVEN4 clinical trials for which patients with CHAwI on emicizumab prophylaxis (at the labeled dose) used rFVIIa. Initial individual dosing with rFVIIa, dosing intervals, and cumulative dosing were evaluated. All AEs reported in each of the 3 trials, including available narratives, were assessed. The cut-off dates for data presented were for HAVEN1 (primary analysis) September 2017; HAVEN2 (interim analysis) October 2017; and HAVEN4 (primary analysis) December 2017.26
eData from a randomized, double-blind, parallel-group, multicenter study of patients with hemophilia A or B with and without an inhibitor. Patients were given NovoSeven® RT at dosing intervals of 2 to 3 hours. Efficacy reflects the number of patients reporting excellent, effective, or partially effective results. Response was rated as “excellent” if a patient demonstrated definitive relief of pain/tenderness and/or if there was a measurable decrease in the size of the bleed (or arrest of bleeding) in 8 hours or less. An “effective” response was measured by any of these 3 events occurring from 8 to 14 hours; a “partially effective” response either occurred after 14 hours or indicated detectable relief of pain/tenderness or decrease in bleeding.29
fPrior to reconstitution, store NovoSeven® RT powder and histidine diluent between 36-77˚F. After reconstitution, store NovoSeven® RT either at room temperature or refrigerated for up to 3 hours. Do not freeze reconstituted NovoSeven® RT or store in syringes.
gBased on ADIVO data collected between 2022 and 2023.30
hData based on postmarketing retrospective safety assessment of clinical trials and registries.31
NovoSeven® RT effectively controls joint bleeds in CHwI—fast.29
Hemostasis was achieved with a median of 2 doses.29,i
Quick readministration
NovoSeven® RT can be readministered as quickly as every 2 hours compared with 6 to 12 hours for FEIBA®1,32
Median 2 doses
A median of 2 doses helped control joint bleeds in as little as 5 hours29,i
Maximum activity
NovoSeven® RT achieved maximum activity within 5-10 minutes of infusion28,j,k
iData from a randomized, double-blind, parallel-group, multicenter study of patients with hemophilia A or B with and without an inhibitor. Patients were given NovoSeven® RT at dosing intervals of 2 to 3 hours. Efficacy reflects the number of patients reporting excellent, effective, or partially effective results. Response was rated as “excellent” if a patient demonstrated definitive relief of pain/tenderness and/or if there was a measurable decrease in the size of the bleed (or arrest of bleeding) in 8 hours or less. An “effective” response was measured by any of these 3 events occurring from 8 to 14 hours; a “partially effective” response either occurred after 14 hours or indicated detectable relief of pain/tenderness or decrease in bleeding.29
jData from a randomized, double-blind trial of healthy subjects (N=22) who received 1 intravenous bolus injection each of NovoSeven® RT and NovoSeven®. Both bolus injections were 90 mcg/kg and occurred 2 to 3 weeks apart at consecutive visits. While the comparison is not shown for FVIIa, activity for NovoSeven® RT was the bioequivalent range of that for NovoSeven® during this period.28
kFVIIa activity IU/mL.28
NovoSeven® RT helps a broad range of patients
with the treatment of bleeding episodes and perioperative management1
Indications
NovoSeven® RT1,l
FEIBA®32,m
OBIZUR®34,n
SEVENFACT®35,o,p
Congenital hemophilia A with inhibitors
Congenital hemophilia B with inhibitors
Acquired hemophiliaq
Congenital factor VII deficiency
Glanzmann's thrombasthenia
with refractoriness to platelet transfusions, with or without antibodies to platelets
= bleed treatment;
= perioperative management
Disclaimer: This chart is not intended to compare efficacy or safety.
lNovoSeven® RT is a recombinant FVIIa.
mFEIBA is an activated prothrombin complex concentrate (aPCC) and is also indicated for prophylaxis in CHAwI and CHBwI patients.
nOBIZUR is a porcine sequence recombinant FVIII.
oSEVENFACT is a recombinant FVIIa.
pSEVENFACT is only indicated for adults and adolescents (12 years and older).
qAdults with acquired hemophilia.
Learn more about how NovoSeven® RT helps your patients with:
Congenital Hemophilia A or B With Inhibitors
Acquired Hemophilia
Congenital Factor VII Deficiency
Glanzmann’s Thrombasthenia With Refractoriness to Platelets

Patients can use MixPro® to reconstitute NovoSeven® RT.
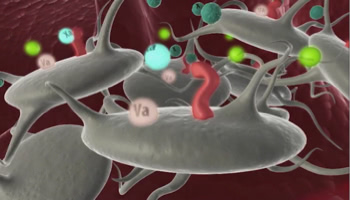
NovoSeven® RT mechanism of action.

Help your patients learn about rare bleeding disorders.
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
References
1. NovoSeven RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
2. Data on file as of 2010. Novo Nordisk Inc; Plainsboro, NJ.
3. Shapiro AD, Neufeld EJ, Blanchette VS, et al. Safety of recombinant activated factor VII (rFVIIa) in patients with congenital hemophilia with inhibitors: overall dose exposure and intervals following >240-mcg/kg doses across trial, registry, and diary studies. Poster presented at: 53rd Annual Meeting and Exposition of the American Society of Hematology; December 10-13, 2011; San Diego, California.
4. Shapiro AD, Gilchrist GS, Hoots WK, et al. Prospective, randomised trial of two doses of rFVIIa (NovoSeven®) in haemophilia patients with inhibitors undergoing surgery. Thromb Haemost. 1998;80(5):773-778.
5. Parameswaran R, Shapiro AD, Gill JC, et al. Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry. Haemophilia. 2005;11:100-106.
6. Ingerslev J, Friedman D, Gastineau D, et al. Major surgery in haemophilic patients with inhibitors using recombinant factor VIIa. Haemostasis. 1996;26:118-123.
7. Rodriguez-Merchan EC, Wiedel JD, Wallny T, et al. Elective orthopedic surgery for hemophilia patients with inhibitors: new opportunities. Semin Hematol. 2004;41(1):109-116.
8. Giangrande PLF, Wilde JT, Madan B, et al. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated): NovoSeven®] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia. 2009;15:501-508.
9. Takedani H, Kawahara H, Kajiwara M. Major orthopaedic surgeries for haemophilia with inhibitors using rFVIIa. Haemophilia. 2010;16:290-295.
10. Boadas A, Fernandez-Palazzi F, De Bosch NB, et al. Elective surgery in patients with congenital coagulopathies and inhibitors: experience of the National Haemophilia Centre of Venezuela. Haemophilia. 2011;17:422-427.
11. Polyanskaya T, Zorenko V, Karpow E, et al. Experience of recombinant activated factor VII usage during surgery in patients with haemophilia with inhibitors. Haemophilia. 2012;18:997-1002.
12. Takedani H, Shima M, Horikoshi Y, et al. Ten-year experience of recombinant activated factor VII use in surgical patients with congenital haemophilia with inhibitors or acquired haemophilia in Japan. Haemophilia. 2015;21:374-379.
13. Balkan C, Karapinar D, Aydogdu S, et al. Surgery in patients with haemophilia and high responding inhibitors: Izmir experience. Haemophilia. 2010;16:902-909.
14. Salaj P, Gurlich R, Svorcova V, et al. Prophylactic preparation and surgical extirpation of a very large abdominal blood cyst in a severe haemophilia A patient with inhibitors managed by rFVIIa. Haemophilia. 2009;15:380-382.
15. Valentino LA, Cooper DL, Goldstein B. Surgical Experience with rFVIIa (NovoSeven®) in congenital haemophilia A and B patients with inhibitors to factors VIII or IX. Haemophilia. 2011;17:579-589.
16. Rodriguez-Merchan EC, Jimenez-Yuste V, Gomez-Cardero P, et al. Surgery in haemophilia patients with inhibitors, with special emphasis on orthopaedics: Madrid experience. Haemophilia. 2010;16:84-88.
17. Caviglia H, Candela M, Galatro G, et al. Elective orthopaedic surgery for haemophilia patients with inhibitors: single centre experience of 40 procedures and review of the literature. Haemophilia. 2011;17:910-919.
18. Banov L, Pavanello M, Piattelli G, et al. Successful urgent neurosurgery management with rFVIIa mega doses in a child with haemophilia A and high titre inhibitor. Blood Coagul Fibrinolysis. 2014;25:518-521.
19. de Souza DG, Waldron PE, Peeler BB, et al. The use of activated factor VII for ventricular septal defect closure in a pediatric patient with hemophilia A and a high titer of inhibitor. J Cardiothorac Vasc Anaesth. 2009;23(5):679-681.
20. Aouba A, Dezamis E, Sermet A, et al. Uncomplicated neurosurgical resection of a malignant glioneuronal tumour under haemostatic cover of rFVIIa in a severe haemophilia patient with a high-titre inhibitor: a case report and literature review of rFVIIa use in major surgeries. Haemophilia. 2010;16:54-60.
21. Goudemand J, Tagariello G, Lopaciuk F. Cases of surgery in highresponder haemophilia patients. Haemophilia. 2004;10(2):46-49.
22. Watts, RG. Successful use of recombinant factor VIIa for emergency fasciotomy in a patient with hemophilia A and high-titer inhibitor unresponsive to factor VIII inhibitor bypassing activity. Am J Haematol. 2005;79:58-60.
23. Rajic N, Savic A, Popovic S, et al. Successful control of bleeding during supracondylar amputation caused by severe compartment syndrome in patient with haemophilia A and high titre of inhibitor. Haemophilia. 2009;15:601-602.
24. Mehta S, Nelson CL, Konkle BA, et al. Total knee arthroplasty using recombinant factor VII in hemophilia-A patients with inhibitors. J Bone Joint Surg Am. 2004;86-A(2):2519-2521.
25. Pruthi RK, Mathew P, Valentino LA, et al. Haemostatic efficacy and safety of bolus and continuous infusion of recombinant factor VIIa are comparable in haemophilia patients with inhibitors undergoing major surgery. Thromb Haemost. 2007;98(4):726-732.
26. Levy GG, Asikanius E, Kuebler P, et al. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: experience from the HAVEN clinical program. J Thromb Haemost. 2019;17(9):1470-1477.
27. National Hemophilia Foundation. Recommendation on the Use and Management of Emicizumab-kxwh (Hemlibra®) for Hemophilia A With and Without Inhibitors, #268. New York, NY: National Hemophilia Foundation; 2022.
28. Bysted BV, Scharling B, Moller T, Hansen BL. A randomized, double-blind trial demonstrating bioequivalence of the current recombinant activated factor VII formulation and a new robust 25°C stable formulation. Haemophilia. 2007;13(5):527-532.
29. Lusher JM, Roberts HR, Davignon G, et al; and rFVIIa Study Group. A randomized, double-blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. Haemophilia. 1998;4(6):790-798.
30. Data on file as of 2023. Novo Nordisk Inc; Plainsboro, NJ.
31. Rajpurkar M, Croteau SE, Boggio L, et al. Thrombotic events with recombinant activated factor VII (rFVIIa) in approved indications are rare and associated with older age, cardiovascular disease, and concomitant use of activated prothrombin complex concentrates (aPCC). J Blood Med. 2019;10:335-340.
32. FEIBA. Package insert. Baxter Healthcare Corporation; 2024.
33. Hedner U. History of rFVIIa therapy. Thromb Res. 2010;125:S4-S6.
34. Obizur. Package insert. Baxter Healthcare Corporation; 2024.
35. SEVENFACT. Package Insert. HEMA Biologics; 2024.
