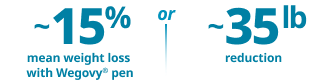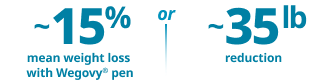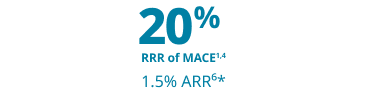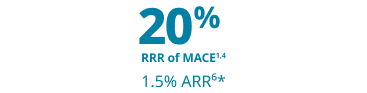In addition to diet and exercise, to reduce the risk of MACE (CV death, non-fatal MI, or non-fatal stroke) in adults with established CVD and either overweight or obesity and for chronic weight management in adults with obesity or overweight with at least one weight-related comorbidity. Click for Limitations of Use.
Resources
Enhance your benefits package with Wegovy®
Remain competitive by giving your employees the option for obesity medicines
Actor portrayals.
Ensure your covered population has access to Wegovy®
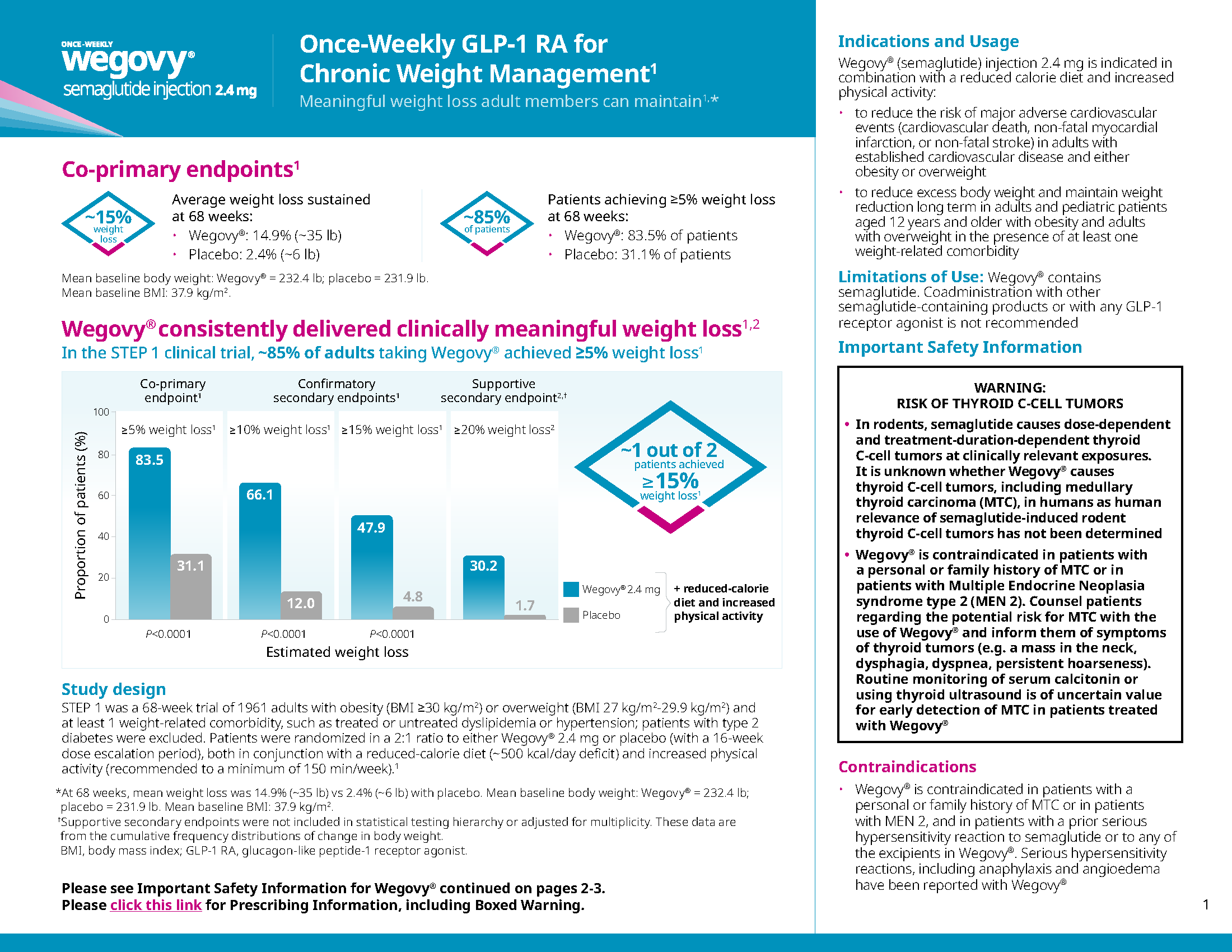
Undeniably powerful weight loss in both the pill and pen1-3
Proven weight-loss efficacy in both pen and pill formats for adults with obesity or overweight with at least one weight-related comorbidity, in addition to diet and exercise


Major cardiovascular event risk reduction that matters
The only GLP-1 RA indicated for weight loss proven to help prevent life-threatening CV events for adults with established CVD and either overweight or obesity, along with diet and exercise1,4,5


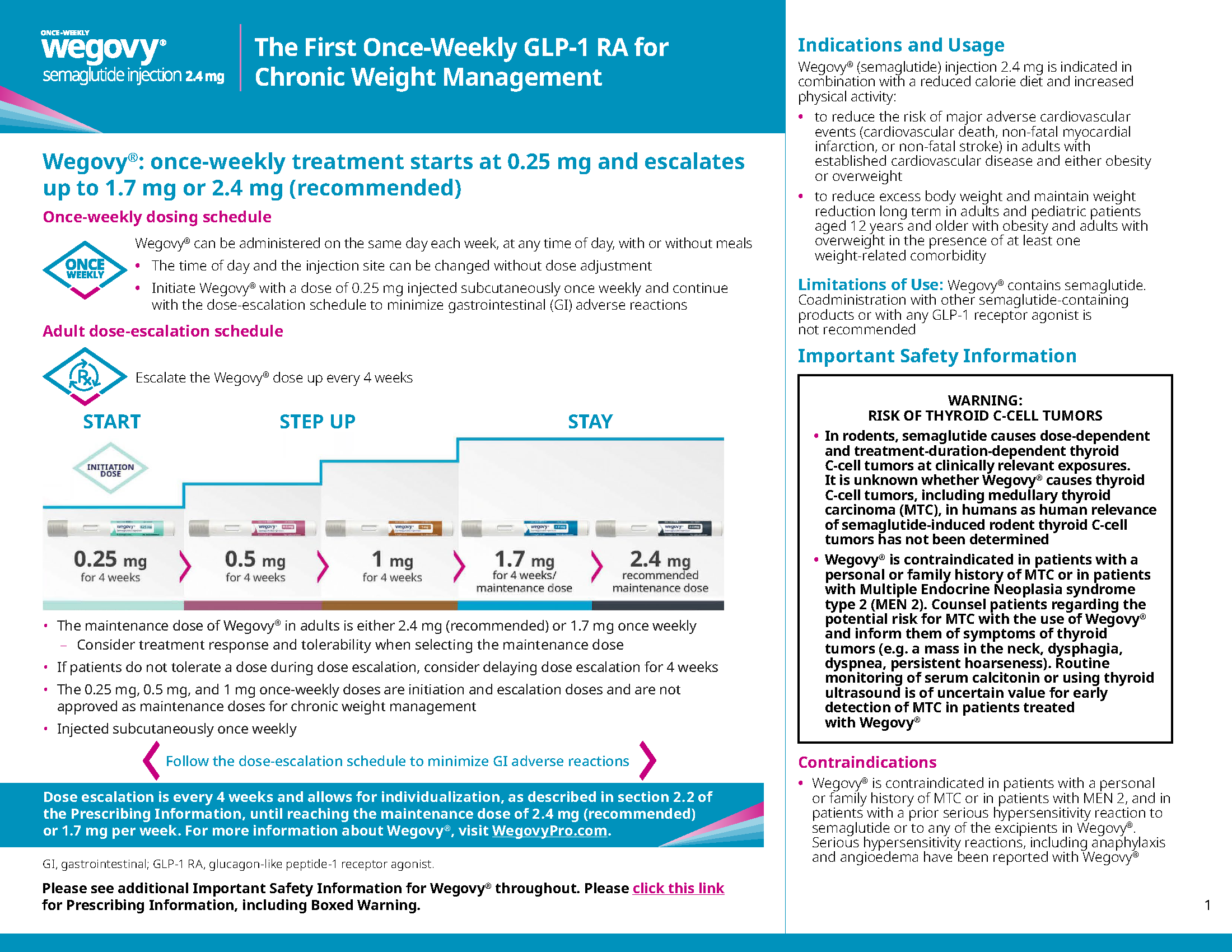
More ways to start than ever before
Available in a once-daily pill or once-weekly pen, with broad access options for your commercially insured population and the lowest Wegovy® self-pay offer yet6
Explore the study information under Efficacy & Safety
CV, cardiovascular; CVD, cardiovascular disease; GLP-1 RA, glucagon-like peptide-1 receptor agonist.
OASIS 4 trial: In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Wegovy®pill achieved significant weight loss1
Analysis of all patients regardless of if they stayed on treatment
Co-primary end point:
Percent change in body weight1*†

Co-primary end point:
Reduction in body weight of ≥5%1*
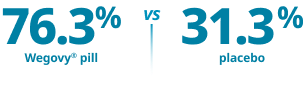
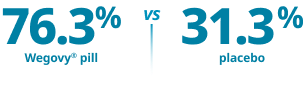
Proportion of patients achieving ≥5% weight loss at 64 weeks*
Confirmatory secondary end points (week 64)1*
≥10% weight loss:
59.8% Wegovy® pill vs 14.4% placebo
≥15% weight loss:
47% Wegovy® pill vs 5.5% placebo
≥20% weight loss:
27.9% Wegovy® pill vs 3.1% placebo
If all patients stayed on treatment
~17% (16.6%) mean weight loss
with Wegovy® pill vs 2.7% with placebo at 64 weeks, in an idealized scenario estimating efficacy if all patients remained on treatment and used no other weight-loss therapies2‡
Mean baseline body weight1: Wegovy®=234.6 lb; placebo=231.1 lb. Mean baseline BMI: 38 kg/m2.
Wegovy® tablets have not been studied for weight reduction in adults with type 2 diabetes and obesity or overweight. The safety and effectiveness of Wegovy® tablets has not been established in pediatric patients.
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).1
*p<0.0001 (unadjusted 2-sided) for superiority.1
†Difference from placebo was -11.2%.1
‡Trial-product estimand: This analysis was not included in the statistical testing hierarchy, and as such, was not controlled for multiplicity.3
BMI, body mass index; RD-MI, retrieved drop-outs multiple imputation.
Study design
OASIS 41,3: A randomized, double-blind, placebo-controlled 64-week trial of 307 adults with obesity (BMI ≥30 kg/m2) or overweight (BMI 27 kg/m2-29.9 kg/m2) and ≥1 weight-related comorbid condition, such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes were excluded. Patients were randomized in a 2:1 ratio to either once-daily Wegovy® pill 25 mg or placebo; both in conjunction with a reduced-calorie diet (~500 kcal/day deficit) and increased physical activity (recommended to a minimum of 150 minutes/week). Discontinuation rate: 18% Wegovy®, 25.5% placebo.
In the 104-week STEP 5 trial, in adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Wegovy® pen delivered significant, sustained weight loss at 2 years2
Analysis of all patients regardless of if they stayed on treatment
Co-primary end point:
Percent change in body weight2
Percentage of patients achieving categorical weight loss at 2 years2¶
Co-primary end point§
≥5% weight loss:
77.1% Wegovy® pen vs 34.4% placebo
Confirmatory secondary end points§
≥10% weight loss:
61.8% Wegovy® pen vs 13.3% placebo
≥15% weight loss:
52.1% Wegovy® pen vs 7% placebo


Supportive secondary end point#
~1 out of 3 patients using the Wegovy® pen achieved


weight loss
36.1% of patients using the Wegovy® pen vs 2.3% of patients taking placebo
#Supportive secondary end points were not included in the statistical hierarchy and, as such, were not controlled for multiplicity.2
If all patients stayed on treatment
~17% (16.7%) mean weight loss
with Wegovy® pen vs 0.6% with placebo at 2 years, in an idealized scenario estimating efficacy if all patients remained on treatment and used no other weight-loss therapies2**
Mean baseline body weight: Wegovy® pen=232.8 lb; placebo=234.8 lb. Mean baseline BMI: 38.5 kg/m2.2
§p<0.0001 (unadjusted 2-sided) for superiority.2
‖Difference from placebo was -12.6%.2
¶Observed data include only patients who had a body weight assessment at week 104 (144 of 152 for Wegovy® pen arm and 128 of 152 for placebo arm) and do not include all randomized patients.2
**Trial-product estimand: This analysis was not included in the statistical testing hierarchy, and as such, was not controlled for multiplicity.2
Study design
STEP 52: A 104-week trial of 304 adults with obesity (BMI ≥30 kg/m2) or with overweight (BMI 27 kg/m2-29.9 kg/m2) and at least one weight-related comorbid condition, such as treated or untreated dyslipidemia or hypertension, cardiovascular disease, or obstructive sleep apnea; patients with diabetes mellitus were excluded. Patients were randomized in a 1:1 ratio to either once-weekly Wegovy® pen 2.4 mg or placebo (with a 16-week dose escalation), both in conjunction with a reduced-calorie diet (~500 kcal/day deficit) and increased physical activity (recommended to a minimum of 150 min/week). Discontinuation rate: 13% Wegovy®, 27% placebo.
Learn about Wegovy® and sustained weight loss
In the SELECT CVOT, in adults with established CVD and either obesity or overweight, without diabetes, when added to CV standard of care,
Wegovy® is proven to reduce the relative risk of MACE by 20% (1.5% ARR*)1,4,6
Primary composite end point: Time to first occurrence of MACE (CV death, non-fatal MI, or non-fatal stroke)1,4
Event incidence
Percent of patients with MACE1,4:


HR, 0.80 (95% CI, 0.72-0.90)
p<0.001, one-sided p-value
The results from SELECT are based on a trial with injectable Wegovy 2.4 mg.
With Wegovy®
the reduction of MACE was not impacted by age, sex, race, ethnicity,
BMI at baseline, or level of renal function impairment1
Study design
SELECT1,4: A multi-national, double-blind, placebo-controlled, event-driven CV outcomes trial of 17,604 adults with a BMI ≥27 kg/m² and established CVD (prior MI, prior stroke, or PAD) assessing superiority of once-weekly Wegovy® 2.4 mg vs placebo (1:1 randomization, with a 16-week dose escalation) for time to first MACE. Both groups received current standard of care, including CV risk factor management and individualized healthy lifestyle counseling (including diet and physical activity); concomitant CV therapies could be adjusted, at the discretion of the investigator, to ensure participants were treated according to the current standard of care for patients with established CVD. Patients with a history of type 1 or type 2 diabetes were excluded. Median duration of follow-up was 41.8 months.
*1.5% ARR at 40 months (mean duration of follow-up).6
ARR, absolute risk reduction; BMI, body mass index; CI, confidence interval; CVOT, cardiovascular outcomes trial; HR, hazard ratio; MACE, major adverse cardiovascular event; MI, myocardial infarction; PAD, peripheral arterial disease; RRR, relative risk reduction; SOC, standard of care.
Learn about Wegovy® and MACE risk reduction
Wegovy® has the experience, evidence, and coverage that may make a difference for your organization
A well-established track record of evidence and experience
20+
phase 3 randomized clinical trials
including the landmark
SELECT CVOT6
28k+
patients studied*†
in clinical trials across US-approved indications, including SELECT CVOT and STEP clinical trial programs.6
*16,558 patients randomized to Wegovy® treatment arms.6
4+
years
of real-world experience with Wegovy®6‡
Wegovy® is available in a once-daily pill or once-weekly pen, providing options to help manage obesity within your organization.1
Wegovy® pill is the first and only oral GLP-1 RA with proven weight-loss efficacy for adults with obesity or overweight with at least one weight-related comorbidity, in addition to diet and exercise.1
Wegovy® is the only obesity medicine with an indication for MACE risk reduction in adults with obesity or overweight and established cardiovascular disease, in addition to diet and exercise. Accessible through Medicare Part D, it offers expanded access for eligible patients.1,7,8
8 of 10§ patients with commercial insurance coverage have access to Wegovy® as an option for prescription weight management, including out-of-pocket cost between $0 and $30.6 A self-pay option is also available.
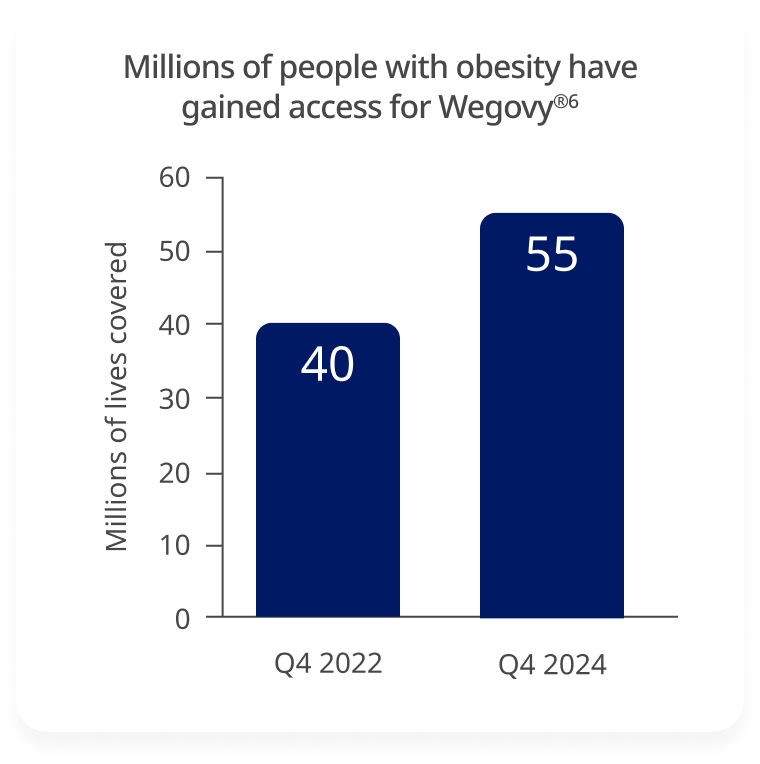
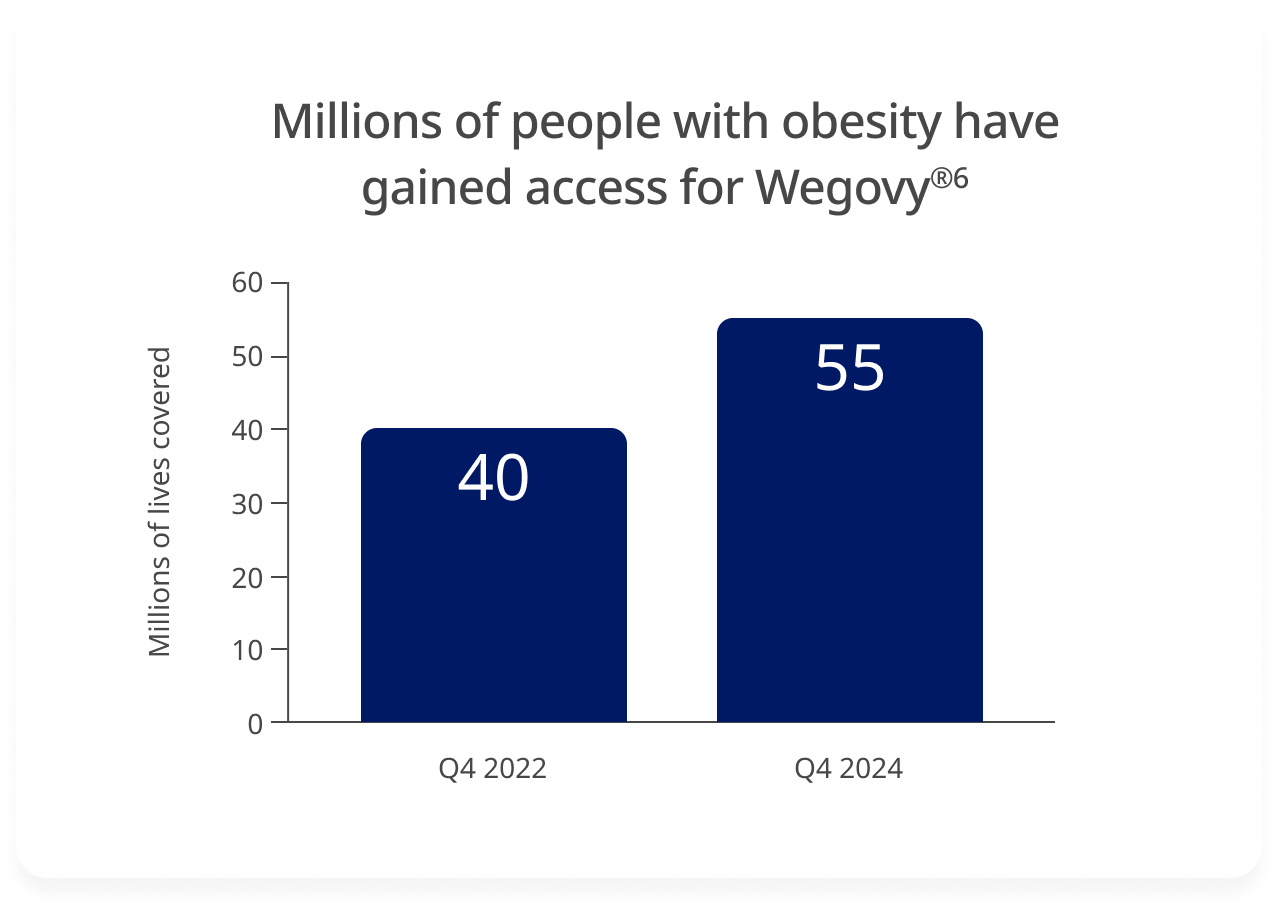
Wegovy® coverage has expanded significantly in 2 years, increasing from 40 million to ~55 million.6
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those less than 12 years of age.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients.
Wegovy® tablets have not been studied for weight reduction in adults with type 2 diabetes and obesity or overweight.
†According to a comprehensive search of completed phase 3 clinical trials registered to clinicaltrials.gov that assessed the number of trials and patients exposed to a GLP-1 RA and/or GLP-1/GIP for weight management.
‡Wegovy® was approved by the FDA on June 4, 2021.9
§Source: IQVIA LAAD Rx for the period of April 2024 to March 2025.6
Statements are based on information licensed from IQVIA for the time periods referenced above, reflecting estimated of real-world activity. All rights reserved.
CV, cardiovascular; CVOT, cardiovascular outcomes trials; GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RA, glucagon-like peptide receptor agonist; MACE, major adverse cardiovascular events.
Download these Novo Nordisk resources that support Wegovy® coverage considerations for employers and payers
Payers and employers now have a wider range of obesity medicine options when building comprehensive weight-management programs10
Employees want GLP-1 obesity medicine benefits
Survey participants in an employee trend report noted a desire to access GLP-1 benefits.11
The results are a compilation of 2 online US-based surveys commissioned by 9am Health in December 2023 (1300 participants) and December 2024 (545 participants).11


use or want to use GLP-1s
75% who aren't taking a GLP-1, but want to, do not have coverage


would stay at a job they didn't like to keep GLP-1 coverage
GLP-1, glucagon-like peptide-1.
Considerations for obesity medicine as part of a comprehensive weight-management benefit
A comprehensive weight-management benefit includes lifestyle interventions, obesity medicines, and surgical treatments12,13
Wegovy® is the only obesity medicine approved to reduce the risk of MACE in appropriate patients1
How to take action for your employees with weight-management options
Take these action steps to assess the impact of obesity on your organization and evaluate the option of obesity medicine coverage. Collaborate with your employee benefits consultant (EBC) or pharmacy benefit manager (PBM) to assess obesity medicine coverage options:
Verify that obesity measurement (ie, BMI, body composition) is integrated into your organization's annual workforce health risk assessment
Measure the impact of obesity and weight-related comorbidities for a comprehensive health profile
Review the prevalence of obesity and related comorbidities within your organization to understand the cost impact
Evaluate your obesity strategy by adding obesity medicine coverage, tracking outcomes, and analyzing medical and pharmacy costs holistically
It’s time to get started with obesity medicine coverage. Start the conversation with your EBC or PBM today.
Register to receive more information as it becomes available.
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
References
- Wegovy® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083-2091.
- Wharton S, Lingvay I, Bogdanski P, et al. Oral semaglutide at a dose of 25 mg in adults with overweight or obesity. N Engl J Med. 2025;393(11):1077-1087.
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232.
- FDA approves first treatment to reduce risk of serious heart problems specifically in adults with obesity or overweight. FDA. Published March 8, 2024. Accessed December 11, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
- Data on file. Novo Nordisk Inc.; Plainsboro, NJ.
- Centers for Medicare & Medicaid Services. CMCS Medicaid and CHIP All State Calls - 2024. Published May 7, 2024. Accessed December 11, 2025. https://www.medicaid.gov/resources-for-states/cmcs-medicaid-and-chip-all-state-calls/cmcs-medicaid-and-chip-all-state-calls-2024/index.html
- HPMS Memos for WK 4 March 18-22 (PDF download: Part D coverage of anti obesity medications with medically accepted indications). Centers for Medicare & Medicaid Services. Published March 20, 2024. Accessed December 11, 2025. https://www.cms.gov/about-cms/information-systems/hpms/hpms-memos-archive-weekly/hpms-memos-wk-4-march-18-22
- FDA approves new drug treatment for chronic weight management, first since 2014. FDA. Published June 4, 2021. Accessed December 11, 2025. https://www.prnewswire.com/news-releases/fda-approves-new-drug-treatment-for-chronic-weight-management-first-since-2014-301306223.html
- Tak YJ, Lee SY. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Mens Health. 2021;39(2):208-221. doi.org/10.5534/wjmh.200010
- 9am Health. Navigating weight loss medications in 2025. How to drive ROI and employee satisfaction. January 2025.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; The Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;24(25 suppl 2):S102-S138. doi:10.1161/01.cir.0000437739.71477.ee
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi:10.4158/EP161365.GL