Indicated for treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital factor VII (FVII) deficiency, Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, and in adults with acquired hemophilia.
MixPro® makes preparing a dose fast for your patients
NovoSeven® RT provides a prefilled syringe with every dose. See reconstitution and administration steps below.
MixPro® makes preparing a dose fast for your patients
NovoSeven® RT provides a prefilled syringe with every dose. See reconstitution and administration steps below.
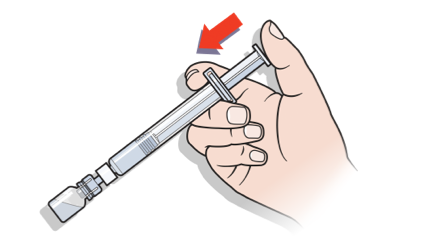
Preparing a dose is as easy as attach, twist, and mix.
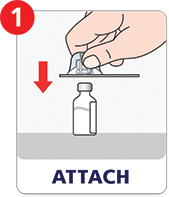
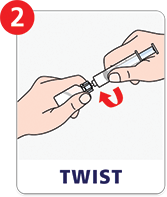

Refer to Instructions for Use for complete information
Administering NovoSeven® RT

Prior to administration
- Inspect the reconstituted NovoSeven® RT visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed
- Do not freeze reconstituted NovoSeven® RT or store it in syringes
- Administer within 3 hours after reconstitution
- Do not mix with other infusion solutions
- Discard any unused solution
Please note, this is not the complete NovoSeven® RT Instructions for Use. Please refer to the Instructions for Use provided with the Prescribing Information.
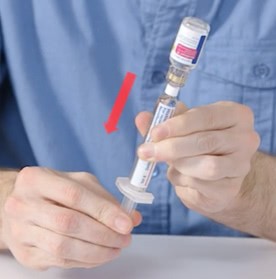
Administration
Administer NovoSeven® RT bolus infusion using the following procedures:
- Administer as a slow bolus injection over 2 to 5 minutes, depending on the dose administered
- If line needs to be flushed before or after NovoSeven® RT administration, use 0.9% Sodium Chloride Injection, USP
- Discard any unused reconstituted NovoSeven® RT after 3 hours
Administer NovoSeven® RT continuous infusion for perioperative management using the following procedures:
- Administer as a continuous infusion at 50 mcg/kg/hr using an infusion pump.
- If line needs to be flushed before or after NovoSeven® RT administration, use 0.9% Sodium Chloride Injection, USP.
Please see the Prescribing Information for further details.
Caution:
- The prefilled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector
- Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior®, Bionector®), and their use can damage the connector and affect administration. To administer product through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe
- If you have encountered any problems with attaching the prefilled histidine diluent syringe to any Luer-lock compatible device, please contact Novo Nordisk at (877) 668-6777
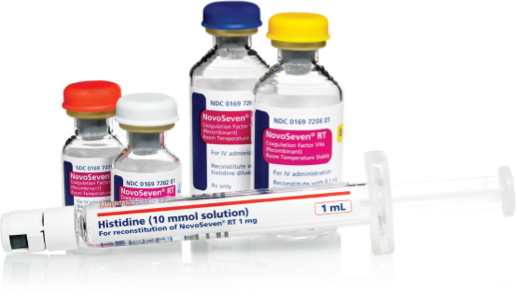
Room temperature stability gives patients rapid access to treatment.
- Room temperature stable up to 77°F1,a
- Available in 1-mg, 2-mg, 5-mg, and 8-mg vials1
- Everything they need to reconstitute is in 1 small box
- Each vial comes with a prefilled syringe, meaning no extra steps to fill a syringe with diluent1,b
aPrior to reconstitution, store NovoSeven® RT powder and histidine diluent between 36-77˚F. After reconstitution, store NovoSeven® RT either at room temperature or refrigerated for up to 3 hours. Do not freeze reconstituted NovoSeven® RT or store in syringes.
bCompared with reconstitution using histidine vials.
Need dosing information?

Find the dose for your patients.*
*As a reminder, for US health care professionals only.
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Reference
1. NovoSeven RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc.

