Indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes. Esperoct® is not indicated for the treatment of von Willebrand disease.
Financial programs and support for patients
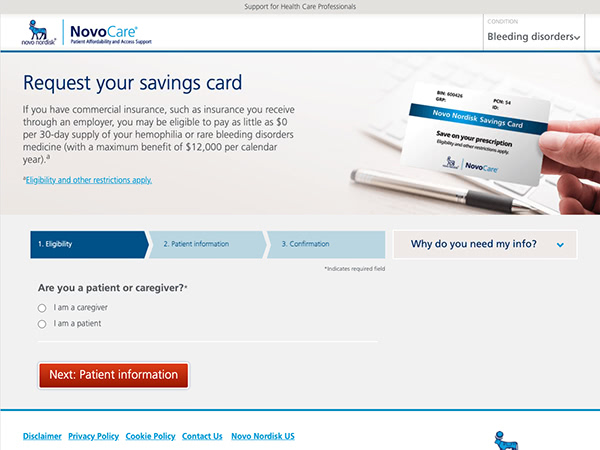
Novo Nordisk provides assistance through NovoCare®, a network to help your patients access co-pay savings, product support, and more.
Financial programs and support for patients
Novo Nordisk provides assistance through NovoCare®, a network to help your patients access co-pay savings, product support, and more.

NovoCare® is designed for your patients
Patients can access:
- Product support programs that help with treatment costs
- Representatives who speak Spanish to better serve them
You can help your patients by calling 1‑844‑668‑6732 or have them contact NovoCare® at NovoCare.com
NovoCare® can help with:
QuickCheck™ benefits verification
Verify your patients' benefits and receive confirmation of coverage. If no coverage is available, names of alternative therapies may be supplied.
Patient Assistance Program (PAP)
See if your patients are eligible to receive their treatment free of charge. No registration or monthly fees required.
Patient Trial
Program
Find out if your patients qualify to receive a limited supply of Novo Nordisk's product for free.
Patient
enrollment form
Download and complete the patient enrollment form to attest your patient's need for treatment.
Interim Product Program
This limited program provides hemophilia therapy to qualifying patients during a coverage gap. Contact a NovoCare® Specialist by calling 1‑844‑668‑6732.
Free Trial Prescription

Help your patients make the switch to Esperoct®. Learn more about Novo Nordisk’s trial prescription and support programs. To learn more about our trial prescription program, please call 1‑844‑668‑6732 to speak with a NovoCare® Specialist.
Questions about the NovoCare® program?
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease