Indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes. Esperoct® is not indicated for the treatment of von Willebrand disease.


For women and girls with hemophilia A, the time has come to understand.
Actor portrayals


![Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] logo](/content/dam/novonordisk/novomedlink/new/brand/hemophilia/esperoct/about/women-in-hemophilia/logo-rybelsus.png)
![Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] logo](/rare-bleeding-disorders/products/treatments/esperoct/about/women-with-hemophilia/_jcr_content/root/slab_1362471607/content/container_1843608870/container/image.img.png/1750965578365/logo-rybelsus.png)
Extended half-life bleed control she deserves1
Would you recognize her hemophilia symptoms?

Erica has consistent easy bruising
A male counterpart might have been tested here2

Heavy menstrual bleedinga
Not knowing what is normal can be a barrier to further investigation3

Joint pain
A male counterpart with the same family history of hemophilia may have been more likely to be tested here, if not tested previously2

Pursuing other diagnoses
When a female hemophilia carrier experiences bleeding, healthcare providers may pursue alternative diagnoses, causing a delay in recognizing the true diagnosis4

Prescribed hormone therapy
Hormone therapy ineffective in resolving symptoms

Hysterectomy recommended
Facing the loss of her fertility, Erica began investigating the role of her family history of hemophilia

Could it be hemophilia?

Hematologist willing to test her factor levels

Erica’s FVIII levels were 30 IU/dL

Diagnosis of hemophilia A

Appropriate treatments prescribed

No HCP willing to test Erica’s factor levels

Failure to properly diagnose

Inadequate symptom management
Patient Diagnostic
Meet Erica
Not a real patient – hypothetical profile
Erica is a 26-year-old teaching assistant who experiences easy bruising, joint pain, and heavy, long menstrual periods.
Father and brother on factor therapy
Hormone therapy for heavy periods that was not helpful
Possibility of hemophilia dismissed by multiple providers
Diagnosis of anemia
A family history of hemophilia A
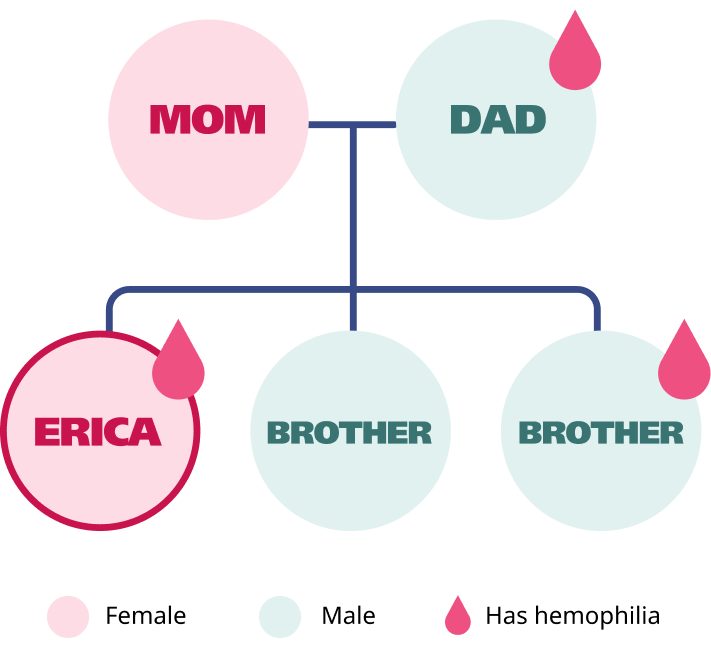
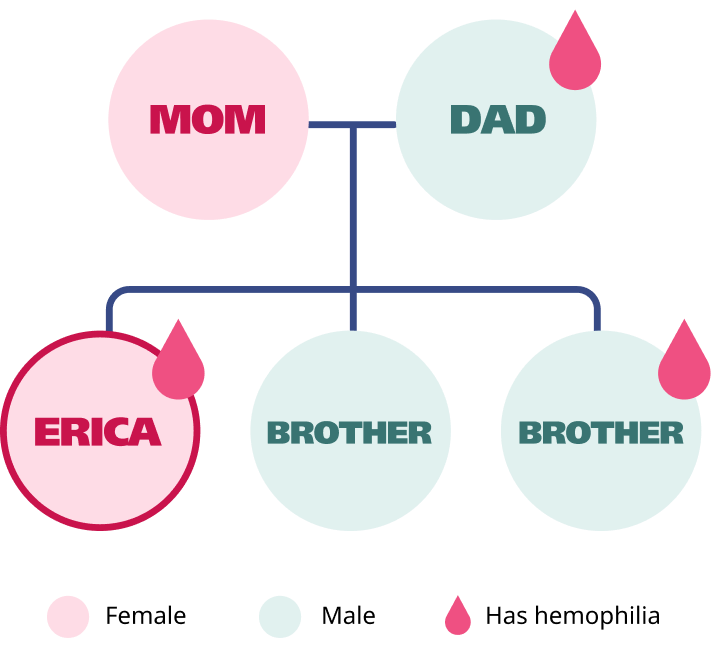
aLonger than 7 days, changing pads/tampons every 2 hours or less, passing blood clots larger than 1 inch or the size of a quarter, requires double protection, or requires changing pad/tampon overnight.5,6
Redefining the clinical approach to women and girls with hemophilia A starts with understanding the signs and symptoms unique to them.
Same hemophilia, unique experiences
Getting Erica the right diagnosis and treatment—sooner
Although World Federation of Hemophilia guidance says that women with hemophilia should be treated the same as men,7 this may not be followed by all providers. Support patients like Erica better by understanding that:
Women can be more than “carriers” of hemophilia4
To improve diagnosis and management, the International Society on Thrombosis and Haemostasis worked with hemophilia experts, patients, and others in its community to define women, girls, and people who have or had the ability to menstruate as persons with hemophilia, not just carriers.8
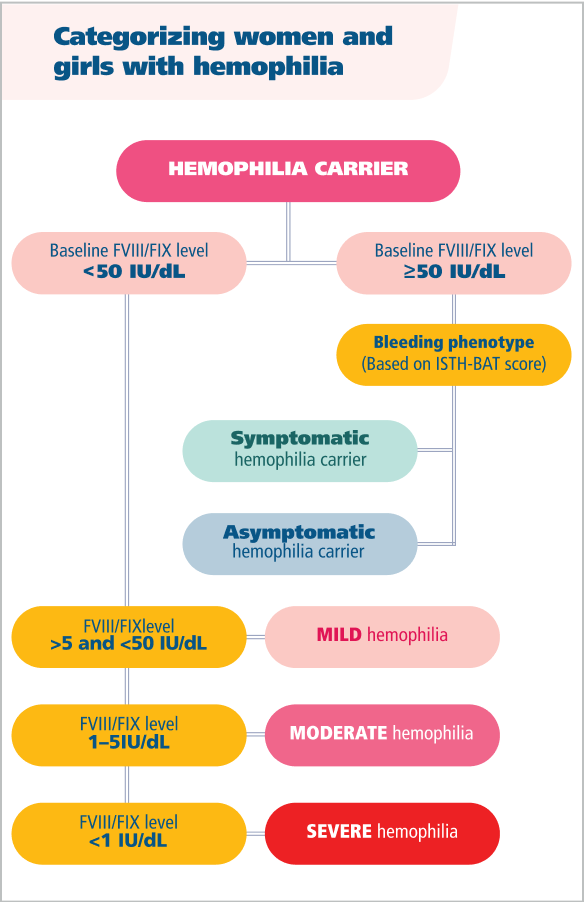
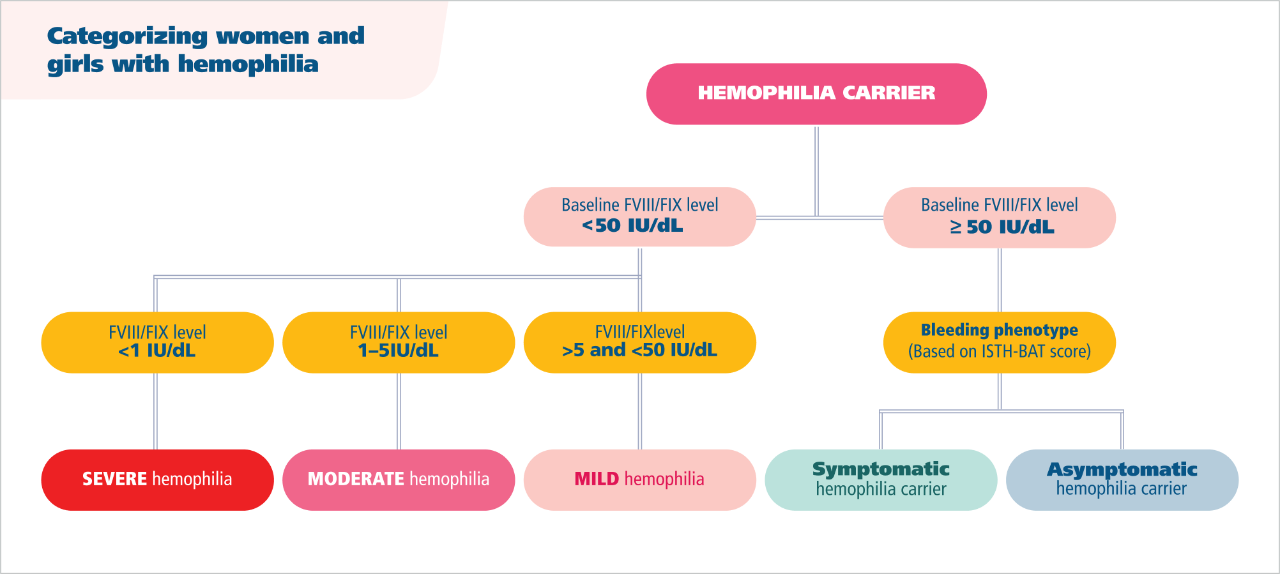
Hemophilia in women and girls is defined by more than just factor levels8
Factor levels can vary over time due to oral contraceptives, infections, inflammation, pregnancy, aging, and other variables.7


Hemophilia affects women and girls in ways unique to them
In one study, nearly 2 of 3 women carriers of hemophilia experienced abnormally heavy menstrual bleeding.9
Actor portrayal
Let’s work together
so that each woman and girl with hemophilia can receive a correct, timely diagnosis and appropriate treatment options.
Esperoct®: Extended half-life bleed control she deserves


View efficacy data on long-term bleed protection
See how broad temperature stability gives patients flexibility
Learn how Esperoct® protects patients of all ages


Intended for use by health care professionals only.
Explore other treatments and get 1:1 support
![Novoeight® [antihemophilic factor (recombinant)] logo and vials](/content/dam/novonordisk/novomedlink/new/brand/hemophilia/esperoct/about/women-in-hemophilia/novoeight-min.png)
![Novoeight® [antihemophilic factor (recombinant)] logo and vials](/rare-bleeding-disorders/products/treatments/esperoct/about/women-with-hemophilia/_jcr_content/root/slab_1485051317/content/container_copy/image.img.png/1750965894618/novoeight-min.png)
Meet Novoeight®
A standard half-life Factor 8 treatment that can be used as prophylaxis or on demand
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease
- Esperoct® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Arya S, Wilton P, Page D, et al. "They don’t really take my bleeds seriously": Barriers to care for women with inherited bleeding disorders. J Thromb Haemost. 2021;19(6):1506-1514.
- Arya S, Wilton P, Page D, et al. "Everything was blood when it comes to me": understanding the lived experiences of women with inherited bleeding disorders. J Thromb Haemost. 2020;18(12):3211-3221.
- Livanou ME, Matsas A, Valsami S, Papadimitriou DT, Kontogiannis A, Christopoulos P. Clotting factor deficiencies as an underlying cause of abnormal uterine bleeding in women of reproductive age: a literature review. Life (Basel). 2023;13(6):1321.
- American College of Obstetricians and Gynecologists. FAQs Abnormal Uterine Bleeding. American College of Obstetricians and Gynecologists; December 2021. Accessed Sept 11, 2025. https://www.acog.org/womens-health/faqs/abnormal-uterine-bleeding.
- James PD. Women and bleeding disorders: diagnostic challenges. Hematology Am Soc Hematol Educ Program. 2020; 2020(1):547-552.
- World Federation of Hemophilia. Women and girls with hemophilia. World Federation of Hemophilia; 2023 Accessed July 2025. https://www1.wfh.org/publications/files/pdf-2342.pdf
- Weyand AC, Sidonio RF Jr, Sholzberg M. Health issues in women and girls affected by haemophilia with a focus on nomenclature, heavy menstrual bleeding, and musculoskeletal issues. Haemophilia. 2022;18(suppl 4):18-25.
- Kadir RA, Economides DL, Sabin CA, Pollard D, Lee CA. Assessment of menstrual blood loss and gynaecological problems in patients with inherited bleeding disorders. Haemophilia. 1999;5(1):40-48. doi:10.1046/j.1365-2516.1999.00285.x