Indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes. Esperoct® is not indicated for the treatment of von Willebrand disease.
What’s included in the Esperoct® package:
6 vial sizes available
Differentiate dose strengths through single-dose vials with color-coded caps.1

Vial adapter
Connects the syringe and vial, with a 25-μm inline particle filter.1

MixPro® prefilled diluent syringe
Latex-free syringe contains 4 mL of 0.9% saline solution—works with all dose strengths.1

Ready in 3 simple steps
These are not the complete Esperoct® Instructions for Use. Please refer to the Instructions for Use provided with the Prescribing Information.
1. Attach
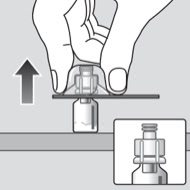
Prefilled diluent syringe contains 4 mL of diluent – works with any dose strength.
2. Twist
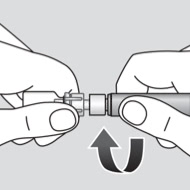
Adapter connects the syringe and vial with a 25 µm inline particle filter.
3. Mix
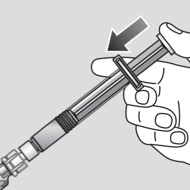
After mixing, the reconstituted solution can be administered.
After mixing, the reconstituted solution can be stored for use within 4 hours at up to 86 °F.1
Preparation
- Always wash hands and ensure that the area is clean before performing the reconstitution procedures
- Use aseptic technique during the reconstitution procedures
- If the dose requires more than one vial of Esperoct® per infusion, reconstitute each vial according to the instructions
Administration: For intravenous infusion only
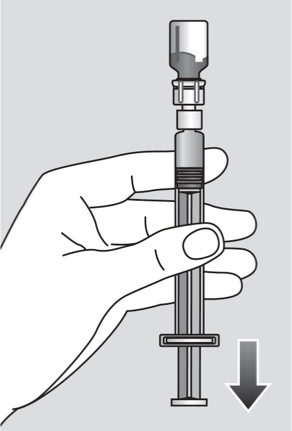
Administer using the following procedure:
- Invert the Esperoct® vial and slowly draw the solution into the syringe.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- Attach the syringe to the luer end of an infusion needle set.
- Infuse the reconstituted Esperoct® intravenously slowly over approximately 2 minutes.
- After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused Esperoct®, and other waste materials.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed
- Do not administer Esperoct® in the same tubing or container with other medicinal products
- Administer the Esperoct® solution immediately. If not used immediately after the reconstitution, store the solution in the vial with the vial adapter and the syringe attached, at room temperature ≤ 86 °F (30 ºC) for ≤ 4 hours, or stored in a refrigerator at 36 °F to 46 °F (2 °C to 8 °C) for ≤ 24 hours
Caution:
- The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
- Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer Esperoct® through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
Please note, these are not the complete Esperoct® Instructions for Use. Please refer to the Instructions for Use included in the Prescribing Information.

For your patients: Starting Esperoct®
Novo Nordisk is here to help your patients with hemophilia A who want to make the switch to Esperoct®. Learn about our trial prescriptiona and product support programs.
To learn more about our trial prescription program, please call 1-844-668-6732 to speak with a NovoCare® Specialist.
aPatients who have been prescribed a Novo Nordisk hemophilia and rare bleeding disorder product for an FDA-approved indication, and who have commercial insurance or who are uninsured, may be eligible to receive a limited supply of free product. Patients who participate in any government, state, or federally funded medical or prescription benefit program, including Medicare, Medicaid, Medigap, VA, DOD, and TRICARE, including patients who participate in a managed Medicaid program or have Medicaid as secondary insurance, are not eligible to receive product support. Product is provided at no cost to the patient or the HCP and is not contingent on any product purchase, and the patient and HCP must not: (1) bill any third party for the free product, or (2) resell the free product.
Count on the clinical trial experience of Esperoct®.
Starting patients on Esperoct®

Novo Nordisk provides resources to help your patient get started on Esperoct®.
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Reference:
- Esperoct [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
