Adult Study Results
Rapid, reliable recovery when every minute counts1
Studied in a comprehensive clinical trial program1
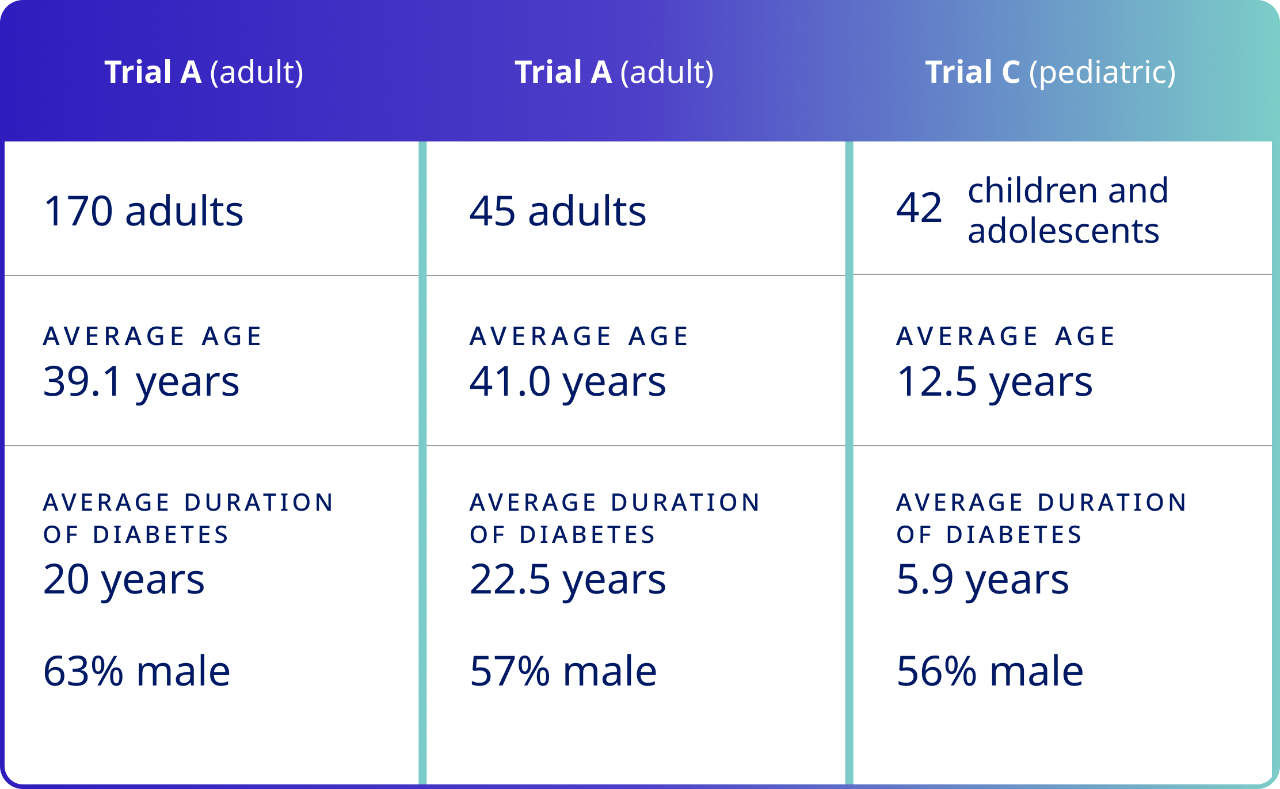
Three randomized, double-blind, placebo-controlled, multicenter clinical trials were conducted in patients with type 1 diabetes.1

In Trials A and B, plasma glucose values were collected and assessed at predose and at 4, 6, 8, 10, 12, 15, 17, 20, 25, 30, 40, 45, 50, 60, 75, and 90 minutes after treatment.1
Trial C assessed plasma glucose values at the same time points as did Trials A and B, with the exception of the 25-, 40-, 50-, 75-, and 90-minute post-treatment time points.1
The primary hypothesis test was superiority of ZEGALOGUE® (dasiglucagon) injection versus placebo.1
In 2 pivotal clinical trials with adult patients, a single dose of ZEGALOGUE® demonstrated rapid recovery of plasma glucose levels versus placebo without additional intervention within 45 minutes1
PRIMARY ENDPOINT: PLASMA GLUCOSE RECOVERY IN ADULT PATIENTS1

TIME TO PLASMA GLUCOSE RECOVERY (TRIAL A)1

In Trial B, 88% of ZEGALOGUE®-treated adult patients recovered in 15 minutes.3
Important Safety Information for ZEGALOGUE®
Contraindications
ZEGALOGUE® is contraindicated in patients with pheochromocytoma because of the risk of substantial increase in blood pressure and in patients with insulinoma because of the risk of hypoglycemia.
Warnings and Precautions
- ZEGALOGUE® is contraindicated in patients with pheochromocytoma because glucagon products may stimulate the release of catecholamines from the tumor. If the patient develops a substantial increase in blood pressure and a previously undiagnosed pheochromocytoma is suspected, 5 to 10 mg of phentolamine mesylate, administered intravenously, has been shown to be effective in lowering blood pressure.
- In patients with insulinoma, administration of glucagon products may produce an initial increase in blood glucose; however, ZEGALOGUE® administration may directly or indirectly (through an initial rise in blood glucose) stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. ZEGALOGUE® is contraindicated in patients with insulinoma. If a patient develops symptoms of hypoglycemia after a dose of ZEGALOGUE®, give glucose orally or intravenously.
- Allergic reactions have been reported with glucagon products; these include generalized rash, and in some cases anaphylactic shock with breathing difficulties and hypotension. Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
- ZEGALOGUE® is effective in treating hypoglycemia only if sufficient hepatic glycogen is present. Patients in states of starvation, with adrenal insufficiency or chronic hypoglycemia may not have adequate levels of hepatic glycogen for ZEGALOGUE® administration to be effective. Patients with these conditions should be treated with glucose.
Adverse Reactions
- The most common adverse reactions (≥2%) associated with ZEGALOGUE® in adults were nausea, vomiting, headache, diarrhea and injection site pain; in pediatrics: nausea, vomiting, headache and injection site pain.
Drug Interactions
- Patients taking beta-blockers may have a transient increase in pulse and blood pressure when given ZEGALOGUE®. In patients taking indomethacin, ZEGALOGUE® may lose its ability to raise blood glucose or may produce hypoglycemia. ZEGALOGUE® may increase the anticoagulant effect of warfarin.
Please click here for ZEGALOGUE® Prescribing Information.
Important Safety Information for ZEGALOGUE®
Contraindications
ZEGALOGUE® is contraindicated in patients with pheochromocytoma because of the risk of substantial increase in blood pressure and in patients with insulinoma because of the risk of hypoglycemia.
Warnings and Precautions
- ZEGALOGUE® is contraindicated in patients with pheochromocytoma because glucagon products may stimulate the release of catecholamines from the tumor. If the patient develops a substantial increase in blood pressure and a previously undiagnosed pheochromocytoma is suspected, 5 to 10 mg of phentolamine mesylate, administered intravenously, has been shown to be effective in lowering blood pressure.
- In patients with insulinoma, administration of glucagon products may produce an initial increase in blood glucose; however, ZEGALOGUE® administration may directly or indirectly (through an initial rise in blood glucose) stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. ZEGALOGUE® is contraindicated in patients with insulinoma. If a patient develops symptoms of hypoglycemia after a dose of ZEGALOGUE®, give glucose orally or intravenously.
- Allergic reactions have been reported with glucagon products; these include generalized rash, and in some cases anaphylactic shock with breathing difficulties and hypotension. Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
- ZEGALOGUE® is effective in treating hypoglycemia only if sufficient hepatic glycogen is present. Patients in states of starvation, with adrenal insufficiency or chronic hypoglycemia may not have adequate levels of hepatic glycogen for ZEGALOGUE® administration to be effective. Patients with these conditions should be treated with glucose.
Adverse Reactions
- The most common adverse reactions (≥2%) associated with ZEGALOGUE® in adults were nausea, vomiting, headache, diarrhea and injection site pain; in pediatrics: nausea, vomiting, headache and injection site pain.
Drug Interactions
- Patients taking beta-blockers may have a transient increase in pulse and blood pressure when given ZEGALOGUE®. In patients taking indomethacin, ZEGALOGUE® may lose its ability to raise blood glucose or may produce hypoglycemia. ZEGALOGUE® may increase the anticoagulant effect of warfarin.
Please click here for ZEGALOGUE® Prescribing Information.
References: 1. Zegalogue® (dasiglucagon). Prescribing information. Zealand Pharma A/S; April 2021. 2. Pieber TR, Aronson R, Hövelmann U, et al. Dasiglucagon: a next-generation glucagon analog for rapid and effective treatment of severe hypoglycemia: results of phase 3 randomized double-blind clinical trial. Diabetes Care. Preprint posted online April 21, 2021. doi:10.2337/DC20-2995 3. Data on file. Zealand Pharma A/S; 2021.