For many patients, T2D is just the beginning1‑3
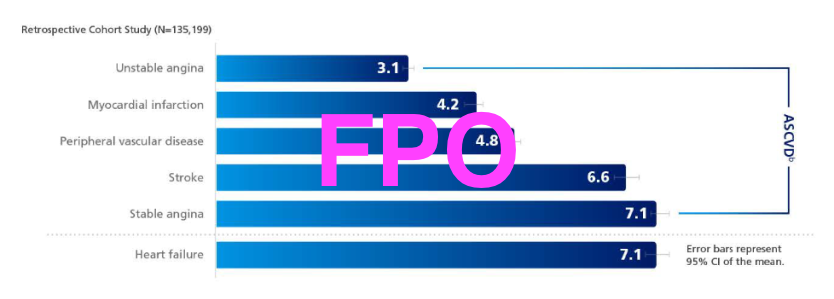
Atherosclerotic cardiovascular disease is the #1 cause of death and disability in patients with T2D4
Higher Risk
People with T2D have a
2-4x Higher
risk of stroke or MI
vs those without T2D5‑7
Earlier onset
Atherosclerotic cardiovascular disease
has been shown to occur
14.6 years earliera
and with a greater mortality risk in people with T2D vs those without4,8,9
Life expectancy
The Emerging Risk Factors Collaboration Study found life expectancy was reduced by up to
19 years
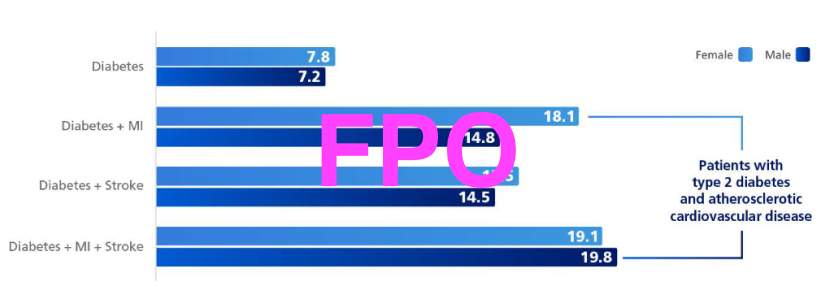
in adults with T2D and atherosclerotic cardiovascular disease10,b,c
AHA/ACC
guidelines
consider patients to have a very high risk for recurrent ASCVD events if they have a history of multiple major ASCVD events or a history of one major ASCVD event+ multiple high-risk conditions, including diabetes11
Consider residual risk in your patients with T2D and ASCVD
aBased on a retrospective cohort study using claims to identify adults with (n=379,003) and without (n=9,018,082) T2D living in Ontario, Canada, on or before April 1, 1994. Individuals were followed up to record CVD events until March 31, 2000.8
bPatients aged 50 years with T2D and a history of MI and stroke and baseline.10
cAn analysis of individual patient data from the Emerging Risk factors Collaboration (n=689,300 years of baseline surveys: 1960-2007) and the UK Biobank, a prospective cohort study (n=499,808; years of baseline surveys: 2006-2010). Patient-level data were analyzed to determine the association of cardiometabolic multimorbidity with mortality and reductions in life expectancy. Cumulative survival was estimated by applying hazard ratios for mortality to contemporary US age-specific death rates.10
ACC=American College of Cardiology; AHA=American heart Association; ASCVD=atherosclerotic cardiovascular disease; CVD=cardiovascular disease; MI=myocardial infarction; T2D=type 2 diabetes.
What are the potential impacts of comorbid disease for patients with T2D?
Patients managing comorbid conditions, such as CKD and CVD, can incur high health care costs in addition to the expenses of diabetes care.12-14
32% of patients with T2D have CVD15,d
ASCVD is the leading cause of morbidity and mortality in patients with T2D16,e
Life expectancy was reduced by up to 19 years in adults with T2D and ASCVD10,f,g
Consider residual CV risk in your patients with CVD and T2Dh
dFrom an international systemic review between January 2007 to March 2017, among 4,549,481 adults aged 18 years or older assessing prevalence of cardiovascular disease in patients with type 2 diabetes.15
eIn adults, T2D accounts for approximately 90%-95% of all diagnosed cases of diabetes in the US according to the Centers for Disease Control and Prevention.17
fPatients aged 50 years with T2D and a history of MI and stroke at baseline.10
gFrom an analysis of individual patient date from the Emerging Rick factors Collaboration (n=689,300; years of baseline surveys: 1960-2007) and the estimates of UK Biobank, a prospective cohort study (n=499,808; years of baseline surveys: 2006-2010). Patient level data were analyzed to determine the associations of cardiometabolic multimorbidity with mortality and reductions in life expectancy. Estimates of cumulative were calculated by applying the hazard ratios (specific to age-at-risk and sex) for cause-specific mortality associated with baseline disease status to US cause-specific death rates.10
hResidual risk refers to the ongoing risk of cardiovascular events that remains even after achieving optimal treatment goals for factors such as LDL cholesterol, blood pressure, and A1C18
~40% of patients with T2D have CKD19
Even early stage CKD increases the risk of CV death1,14
CKD escalates the risk of CV morbidity and mortality as eGFR declines and UACR increases1,14
Consider CV and renal risk in your patients with CKD and T2D
Reasons to choose GLP-1 RAs vs insulin23
GLP-1 RAs, along with healthy lifestyle behaviors, are a recommended option for adults with T2D in need of additional A1C control:
As a high-efficacy option to achieve glycemic goals, including for those patients with a need to address weight concerns23
As an option prior to basal insulin for most adult patients with T2D who need greater efficacy for glycemic control23
In patients with T2D and established atherosclerotic cardiovascular disease
When further intensification for glycemic control is required, consider treating with both a GLP-1 RA and an SGLT-2i with proven CV benefit (label indication)23,m,n
mAccording to the ADA, GLP-1 RAs with proven CVD benefit in adults with T2D have demonstrated an effect on MACE risk reduction in a CVOT include those with an FDA label indication for MACE (CV death, nonfatal MI, or nonfatal stroke) risk reduction.23
nAn SGLT-2i with proven CVD benefit is also an option for patients with established CVD. Please refer to the ADA Standards of Care in Diabetes—2015 for full recommendations.23
For patients with T2D and CVD and/or CVD
Clinical guidelines support a comprehensive approach to T2D management20
The 2022 ADA/EASD consensus report identified 4 key cornerstones that current T2D treatment should address: hyperglycemia, weight, CV risk factor management, and cardiorenal protection20
Major medical societies support use of a GLP-1 RA indicated to reduce the risk of CV events1,14,20-22,i
American Diabetes Association
American Association of Clinical Endocrinology
American College of Cardiology
American Heart Association
Guidelines recommend starting CKD screening at diagnosis of T2D, since evidence of CKD is often already apparent at this time23
iAccording to the ADA, GLP-1 RAs with proven CVD benefit in adults with T2D that have demonstrated an effect on MACE risk reduction in a CVOT include those with an FDA label indication for MACE (CV death, nonfatal MI, or nonfatal stroke) risk reduction.24
ACC=American College of Cardiology; ADA=American Diabetes Association; ASCVD=atherosclerotic cardiovascular disease; CV=cardiovascular; CVD=cardiovascular disease; CKD=chronic kidney disease; CVOT=cardiovascular outcomes trial; EASD=European Association for the Study of Diabetes; GLP-1 RA=glucagon-like peptide-1 receptor agonist; KDIGO=Kidney Disease Improving Global Outcomes; MACE=major adverse cardiovascular event; MI=myocardial infarction; SGLT-2i=sodium-glucose cotransporter-2 inhibitor; T2D=type 2 diabetes.
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
- Ndumele CE, Neeland IJ, Tuttle KR, et al. A synopsis of evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. 2023; 148(20): 1636-1664.
- Arnold SV, Kosiborod M, Wang J, Fenici P. Gannedahl G, LoCasale RJ. Burden of cardio-renalmetabolic conditions in adults with type 2 diabetes within the Diabetes Collaborative Registry. Diabetes Obes Me tab. 2018;20(8):2000-2003.
- Wetmore JB, Li S, Ton TGN, et al. Association of diabetes-related kidney disease with cardiovascular and non-cardiovascular outcomes: a retrospective cohort study. BMC Endocr Disord. 2019; 19(1):89.
- Low Wang CC, Hess CN, Hiatt WR, Golfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus-mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459-2502.
- Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422-1426.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495-2499.
- Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444-470.
- Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368(9529):29-36.
- Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720-1732.
- Di Angelantonio E, Kaptoge S, Wormser D, et al; Emerging Risk Factors Collaboration. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1 ):52-60.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHNACC/AACVPR/AAPNASBCA/ACPM/ADNAGS/ APhNASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209.
- Nichols GA, Amitay EL, Chatterjee S, Steubl D. Health care costs associated with the development and combination of cardio-renal-metabolic diseases. Kidney360. 2023;4(10):1382-1388.
- Fried L, Schmedt N, Folkerts K, et al. High unmet treatment needs in patients with chronic kidney disease and type 2 diabetes: real-world evidence from a US claims database. Nephrol Dial Transplant. 2023;38(3):630-643.
- De Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075-3090.
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17{1):83.
- American Diabetes Association Professional Practice Committee. Cardiovascular disease and risk management: standards of care in diabetes-2025. Diabetes Care. 2025;48(suppl 1):S207-S238.
- US Centers for Disease Control and Prevention. Diabetes basics. 2024. May 15, 2024. Accessed December 16, 2014. https:///www.cdc.gov/diabetes/about/index.html
- Wong ND, Zhao Y, Quek RGW, et al. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: the multi-ethnic study of atherosclerosis. J Clin Lipidol. 2017;11(5):1223-1233.
- von Scholten BJ, Kreiner FF, Rasmussen S, Rossing P, ldorn T. The potential of GLP-1 receptor agonists in type 2 diabetes and chronic kidney disease: from randomised trials to clinical practice. Ther Adv Endocrinol Metab. 2022;13:20420188221112490.
- Davies M, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11 ):2753-2786.
- Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145.
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm-2023 update. Endocr Pract. 2023;29(5):305-340.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4S):S117-S314.
- American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2025. Diabetes Care. 2025;48(suppl 1):S181-S206.