In addition to diet and exercise, to reduce risk of MACE (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CVD and either overweight or obesity, and for chronic weight management in patients with obesity ≥12 years and adults with overweight with at least one weight-related comorbidity. Click for Limitations of Use.
Resources
The Health Impact of Obesity and Overweight
Obesity and overweight are serious diseases that can have serious health risks and complications for your patients1
Learn what are the health risks and impacts, how metabolic adaptation plays a role in losing and keeping weight off, and why it’s important to talk to your patients about obesity
Obesity and overweight are part of a continuum of chronic disease1
Lower BMI doesn’t always mean low risk2,3
Patients living with overweight can still have elevated presence of weight-related comorbidities, with both the duration and location (eg, visceral adiposity) as important determinants of risk.4,5
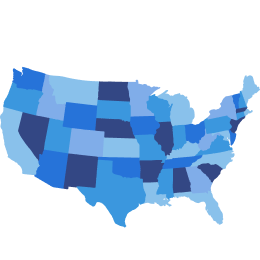
Both projected to increase in prevalence: ~80% US adults (≥25 years) could be living with obesity or overweight by 20506*
*A modeling approach was used to derive projections up to 2050, assuming continuation of trends from 1990 to 2021. Estimates are for adults aged ≥25 years.6
†Data from a UK-based study, including data from the CPRD GOLD database from 2000-2010. A higher prevalence of weight-related comorbidities was reported among adults with overweight than among participants with a normal BMI.2
‡In a nationally representative cohort (>6,000 adults), experiences of weight discrimination were associated with greater odds of becoming obese (2.54x) and of remaining obese among those already obese (3.20x) at a 4-year follow-up.7
BMI, body mass index.
Obesity and overweight are both associated with an increased risk of serious health conditions9,10
Some of the more than 60 complications linked with obesity include3,5:
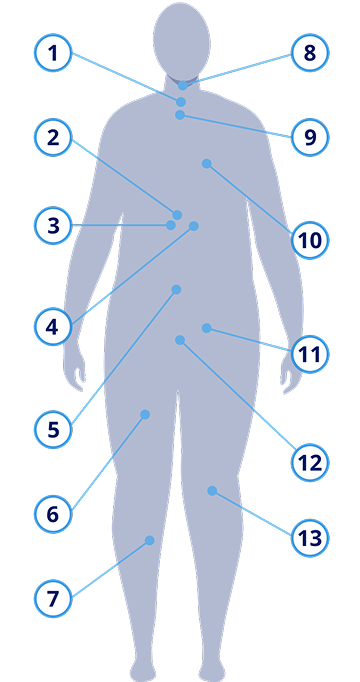
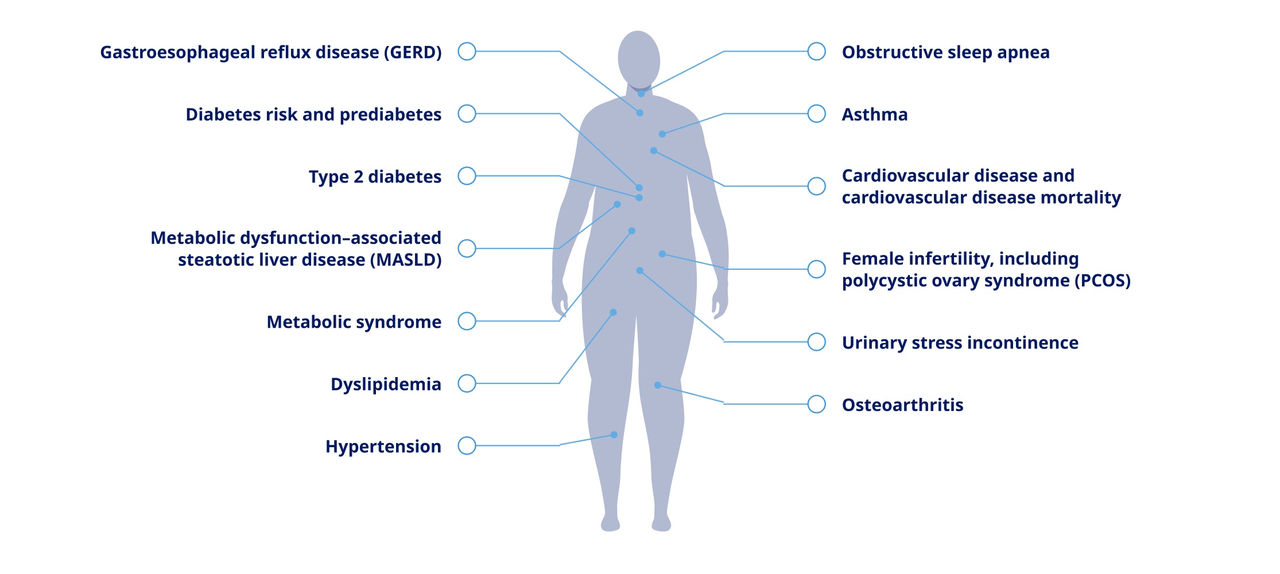
Gastroesophageal reflux disease (GERD)
Diabetes risk and prediabetes
Type 2 diabetes
Metabolic dysfunction–associated steatotic liver disease (MASLD)
Metabolic syndrome
Dyslipidemia
Hypertension
Obstructive sleep apnea
Asthma
Cardiovascular disease and cardiovascular disease mortality
Female infertility, including polycystic ovary syndrome (PCOS)
Urinary stress incontinence
Osteoarthritis
The presence of these complications signal that patients should be evaluated for obesity or overweight3
Sustained weight maintenance is impacted by both internal and external factors11
Biological responses to the gut and brain interaction can contribute to challenges of maintaining weight loss12
The process of metabolic adaptation11,13
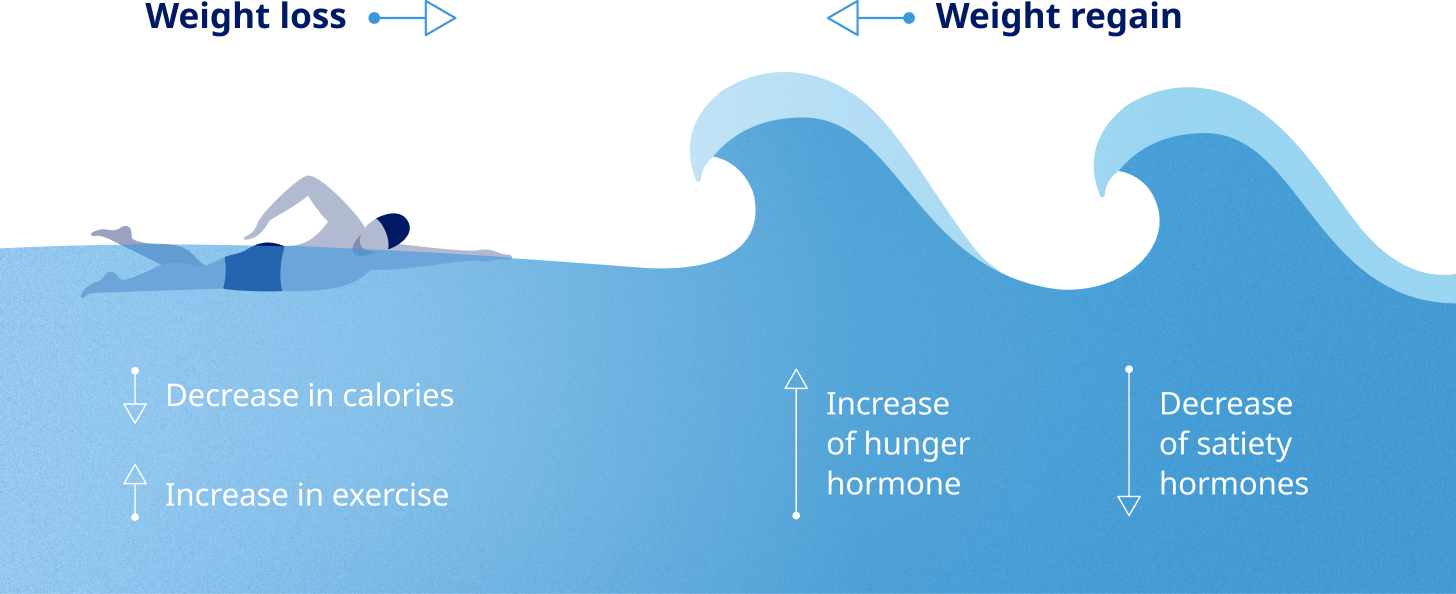
Healthy eating and physical activity are often not enough for lasting weight loss. The biological response of metabolic adaptation can make weight management more difficult.13
Your patients are relying on you to start the obesity conversation14
Evidence shows patient adherence improves when they have a treatment option that meets their needs15
There’s room to improve current approaches when considering anti-obesity medications as an option to support long-term weight management for appropriate patients. Your involvement in your patients’ weight-loss journeys can help and may lead to greater weight loss.16,17*
<20%
of individuals (n=13,158) with overweight reported receiving weight-management counseling to control or lose weight from a health care professional in the previous 12 months18†
*A randomized, controlled, behavioral weight-loss trial with 415 participants that evaluated the mean difference in weight from baseline to 24 months, using questionnaires about patient-provider relationship and satisfaction.17
†Based on a cross-sectional analysis of 4 cycles of the National Health and Nutrition Examination Survey (NHANES) data from 2011-2018 that included respondents (≥18 years) who reported seeing a health care professional at least once in the 12 months prior to data collection.18
‡Based on NHANES data from 2003-2006 of nonpregnant adults (≥20 years) with obesity or overweight. Weight misperception (the predictor variable) was determined among those who reported themselves to be “underweight” or “about the right weight.”19
§Data from 2018-2023, among adults eligible for AOMs (BMI ≥30 kg/m2 or 27 kg/m2-29.9 kg/m2 with ≥1 ORC) in the Mass General Brigham health care system.20
AOM, anti-obesity medication; BMI, body mass index; ORC, obesity-related complication.
Learn more about the weight loss your patients can achieve with a GLP-1 RA
GLP-1 RA, glucagon-like peptide-1 receptor agonist.
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
References
- Rubino F, Cummings DE, Eckel RH, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025;13(3):221-262.
- Haase CL, Eriksen KT, Lopes S, Satylganova A, Schnecke V, McEwan P. Body mass index and risk of obesity-related conditions in a cohort of 2.9 million people: evidence from a UK primary care database. Obes Sci Pract. 2020;7(2):137-147.
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102-S138.
- Cuda S, O'Hara V, Browne NT, et al. Obesity algorithm 2025. Obesity Medicine Association. Accessed December 9, 2025. https://obesitymedicine.org/resources/obesity-algorithm/
- GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298.
- Lee KM, Hunger JM, Tomiyama AJ. Weight stigma and health behaviors: evidence from the Eating in America Study. Int J Obes (Lond). 2021;45(7):1499-1509.
- National Institute of Diabetes and Digestive and Kidney Diseases. Factors affecting weight & health. Updated May 2023. Accessed December 9, 2025. https://www.niddk.nih.gov/health-information/weight-management/adult-overweight-obesity/factors-affecting-weight-health
- National Institute of Diabetes and Digestive and Kidney Diseases. Definition & facts for adult overweight & obesity. Updated May 2023. Accessed December 9, 2025. https://www.niddk.nih.gov/health-information/weight-management/adult-overweight-obesity/health-risks
- Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618-637.
- Lam YY, Ravussin E. Analysis of energy metabolism in humans: a review of methodologies. Mol Metab. 2016;5(11):1057-1071.
- Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699-713.
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604.
- Ananthakumar T, Jones NR, Hinton L, Aveyard P. Clinical encounters about obesity: systematic review of patients’ perspectives. Clin Obes. 2020;10(1):e12347.
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Accessed December 9, 2025.
- Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170-192.
- Bennett WL, Wang NY, Gudzune KA, et al. Satisfaction with primary care provider involvement is associated with greater weight loss: results from the practice-based POWER trial. Patient Educ Couns. 2015;98(9):1099-1105.
- Greaney ML, Cohen SA, Xu F, Ward-Ritacco CL, Riebe D. Healthcare provider counselling for weight management behaviours among adults with overweight or obesity: a cross-sectional analysis of National Health and Nutrition Examination Survey, 2011-2018. BMJ Open. 2020;10(11):e039295.
- Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nutr Phys Act. 2011;8:20.
- Ostrominski JW, Wagholikar KB, Olsson K, et al. Contemporary treatment patterns of overweight and obesity: insights from the Mass General Brigham health care system. Obesity (Silver Spring). 2025;33(2):365-384.