In addition to diet and exercise, to reduce risk of MACE (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CVD and either overweight or obesity, and for chronic weight management in patients with obesity ≥12 years and adults with overweight with at least one weight-related comorbidity. Click for Limitations of Use.
Efficacy & Safety
Well-Studied Safety Profile
Actor portrayals.
Learn more about either Wegovy® pen or pill by selecting the tab below.
Choose Safety Profile
In adults with obesity or overweight with ≥1 weight-related comorbidity,
A well-studied safety profile: Pooled safety data from 3 weight-management clinical trials1
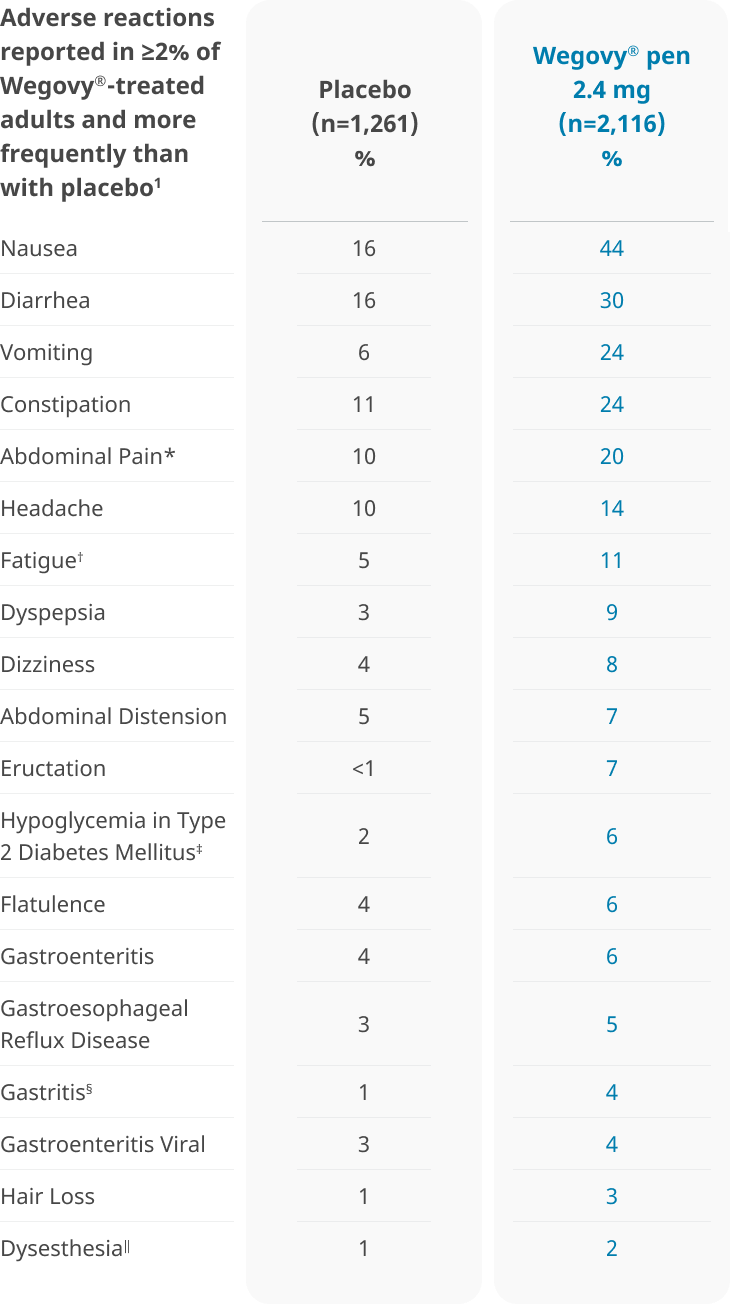
- 6.8% of patients treated with Wegovy® and 3.2% of patients treated with placebo permanently discontinued treatment as a result of adverse reactions1
- Permanent discontinuation of treatment as a result of a gastrointestinal adverse reaction occurred in 4.3% of patients treated with Wegovy® vs 0.7% of patients treated with placebo1
- The most common adverse reactions leading to discontinuation were: Nausea (1.8% vs 0.2%), vomiting (1.2% vs 0%), and diarrhea (0.7% vs 0.1%) for Wegovy® and placebo, respectively1
*Includes abdominal pain, abdominal pain upper, abdominal pain lower, gastrointestinal pain, abdominal tenderness, abdominal discomfort, and epigastric discomfort.1
†Includes fatigue and asthenia.1
‡Defined as blood glucose <54 mg/dL with or without symptoms of hypoglycemia or severe hypoglycemia (requiring the assistance of another person) in patients with type 2 diabetes mellitus not on concomitant insulin (STEP 2, Wegovy® n=403, placebo n=402).1
§Includes chronic gastritis, gastritis, erosive gastritis, and reflux gastritis.1
∥Includes paresthesia, hyperesthesia, burning sensation, allodynia, dysesthesia, skin burning sensation, pain of skin, and sensitive skin.1
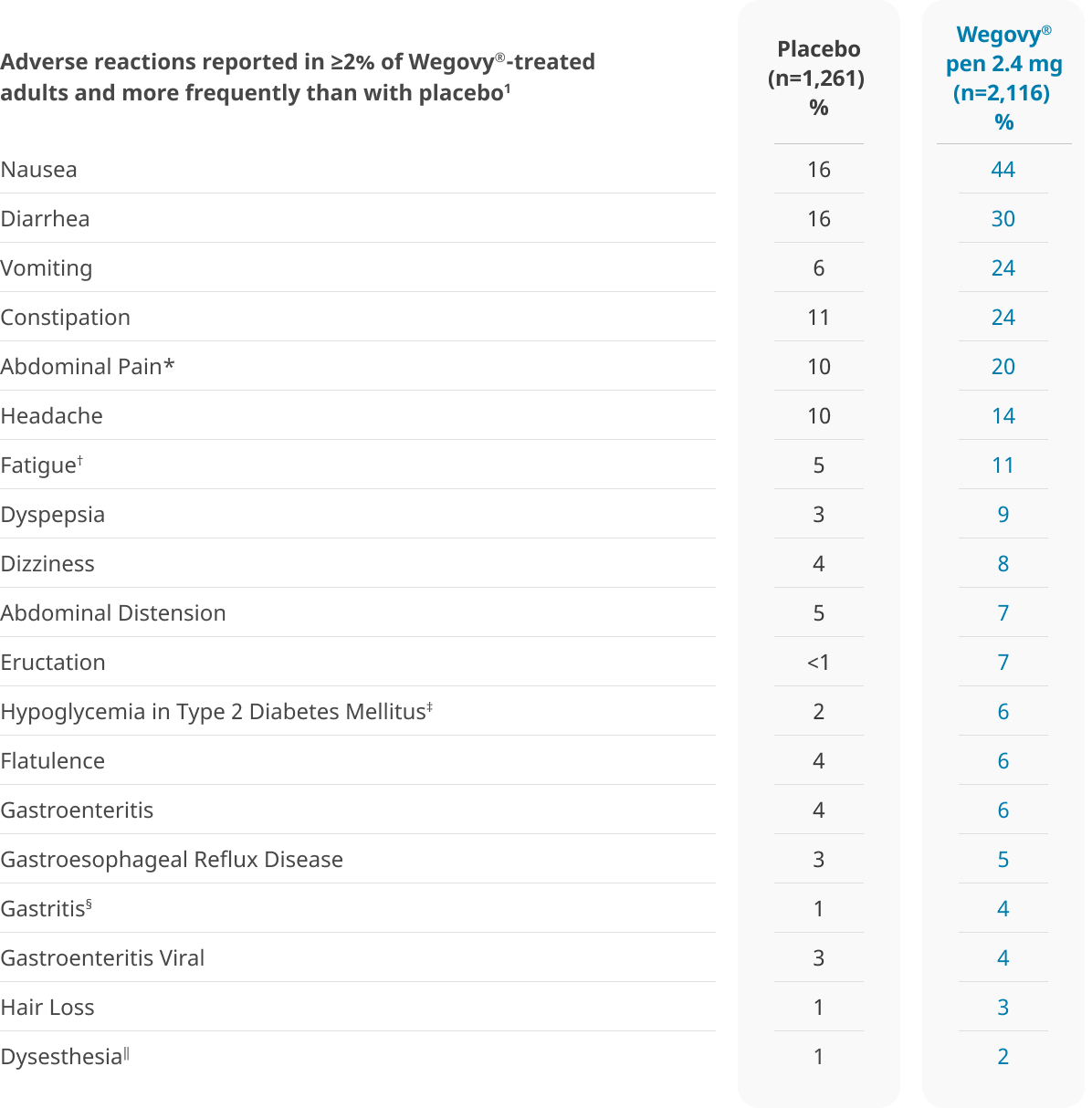
Nausea was generally mild or moderate and transient2
Most occurrences of nausea did not lead to permanent discontinuation of Wegovy®
STEP 1: Reported nausea (median duration 8 days)3
Nausea was not prospectively measured at each patient visit in clinical trials.
In STEP 4: ~9 out of 10 patients achieved the 2.4 mg dose at week 201
- At week 20, 89% of patients achieved the 2.4 mg dose and were randomized, while 11% did not continue in the trial. The most common reason was adverse reactions (n=48; 5.3%)1
In clinical trials, nausea was reported in both treatment arms and was most prevalent during the dose-escalation period1
Choose Safety Profile
In adults with established CVD either overweight or obesity,
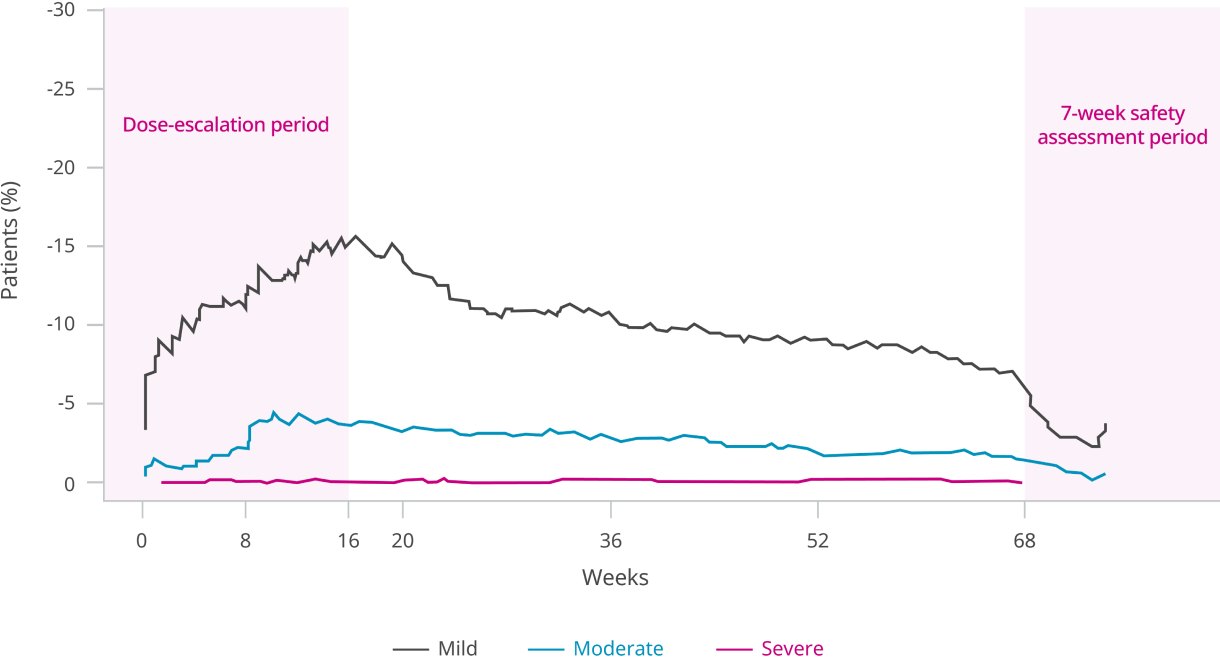
Safety data from the SELECT trial
Safety was evaluated in a trial of 17,604 patients5
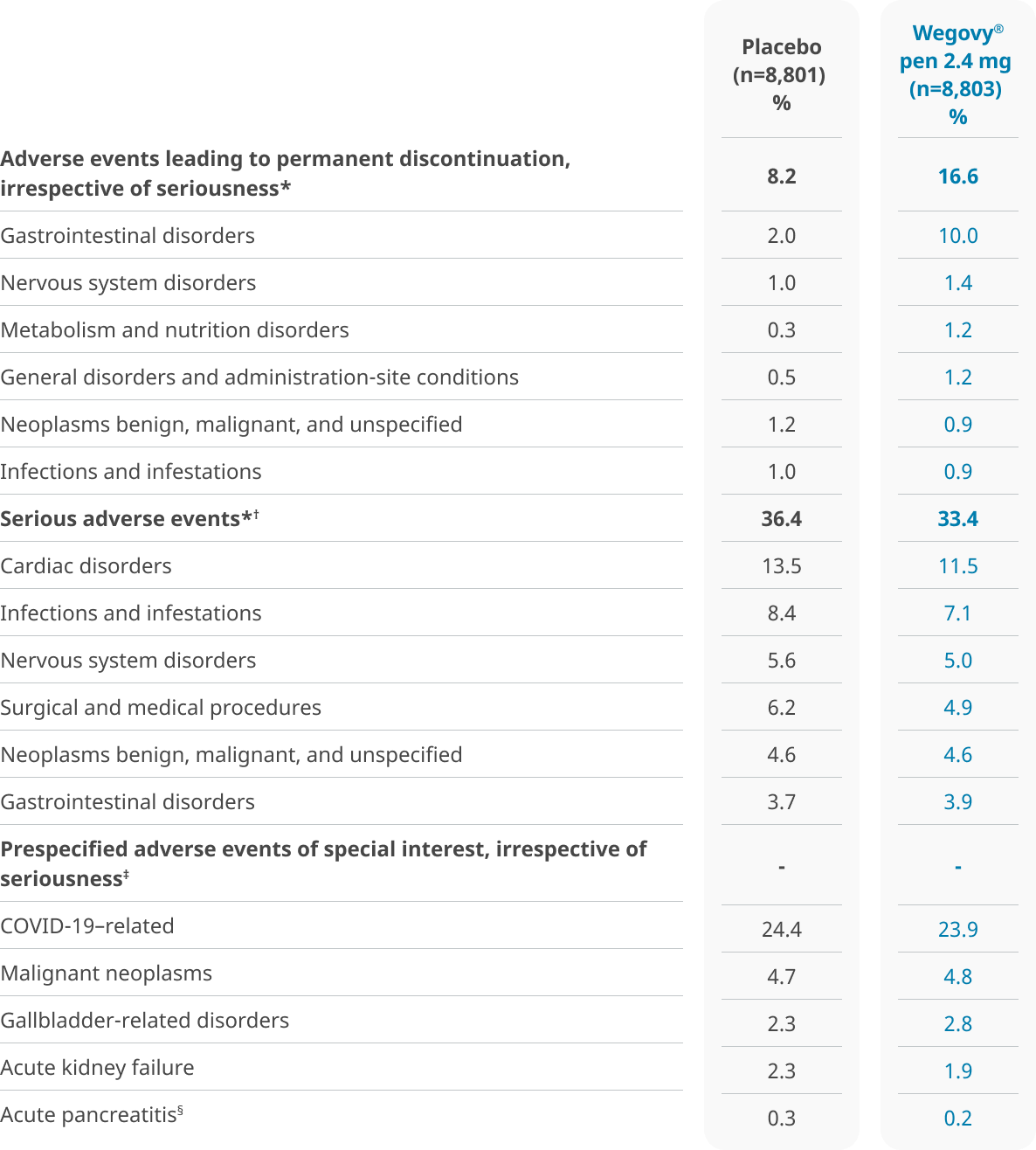
Safety data collection was limited to serious adverse events (including death), adverse events leading to discontinuation, and adverse events of special interest.5
*Events are listed according to system organ class.5
†A serious adverse event is one that has at least one of the following outcomes: Results in death, is life threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent disability/incapacity, is a congenital anomaly/birth defect, or involves an important medical event.5
‡The adverse events of special interest were based on prespecified MedDRA queries.5
§Acute pancreatitis events recorded here are those that were confirmed by the events adjudication committee. Investigators reported pancreatitis (acute or other type) events in 29 patients (0.3%) in the Wegovy® group and 30 patients (0.3%) in the placebo group.5
Choose Safety Profile
Adverse reactions reported in ≥3% of Wegovy® injection-treated adolescent patients aged 12 years and older with obesity and more frequently than with placebo1
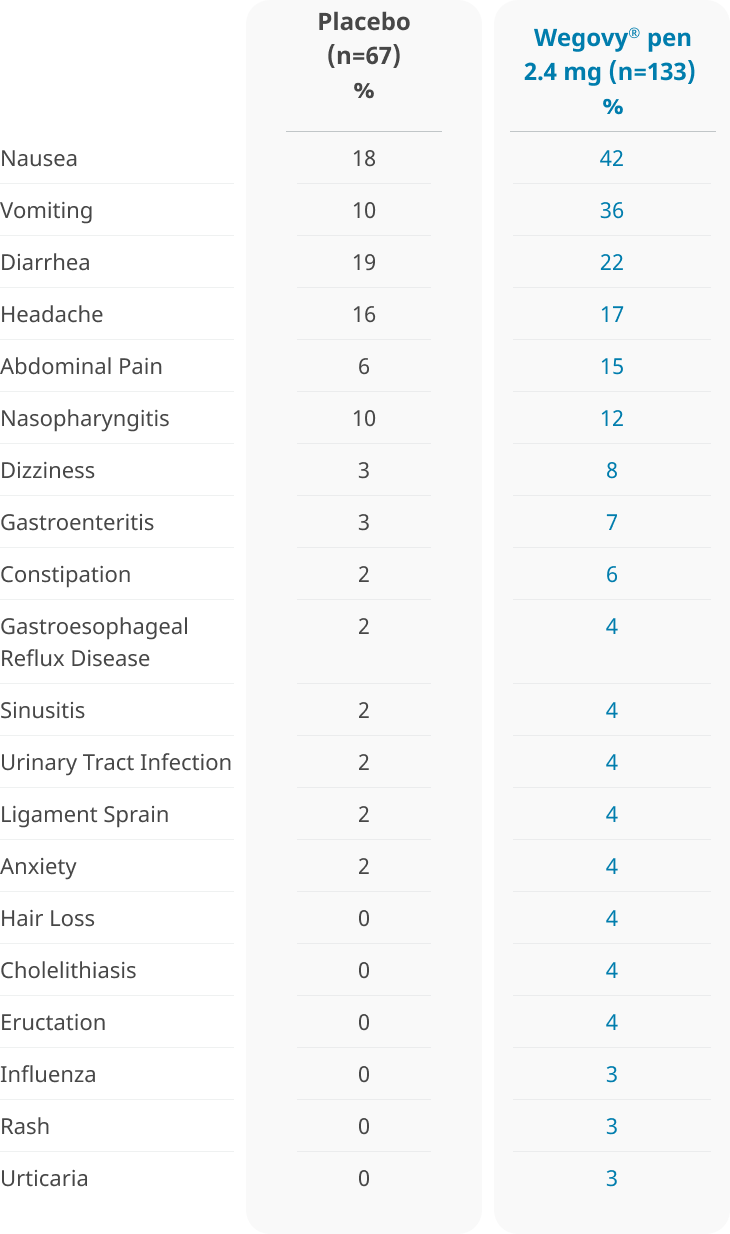
- Gastrointestinal disorders were the most frequent adverse events with Wegovy® (62% vs 42% with placebo)
- 2.3% of patients treated with Wegovy® vs 1.5% of patients who received placebo discontinued treatment as a result of gastrointestinal adverse events6
- Mean increases in resting heart rate of 1.2 beats per minute from baseline were observed with patients taking Wegovy® vs a decrease of 2.3 beats per minute with placebo6
- Adverse reactions with Wegovy® treatment in pediatric patients aged 12 years and older were similar to those reported in adults. Pediatric patients aged 12 years and older treated with Wegovy® had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®1
There are insufficient data in pediatric patients with type 2 diabetes treated with Wegovy® for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® treatment similar to that reported in adults. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia. In pediatric patients aged 12 years and older with type 2 diabetes, monitor blood glucose prior to starting Wegovy® and during Wegovy® treatment. When initiating Wegovy® in pediatric patients aged 12 years and older with type 2 diabetes, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia.1
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those less than 12 years of age.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients.
Well-tolerated safety profile1
The types and frequency of common adverse reactions were similar to the Wegovy® pen1,4
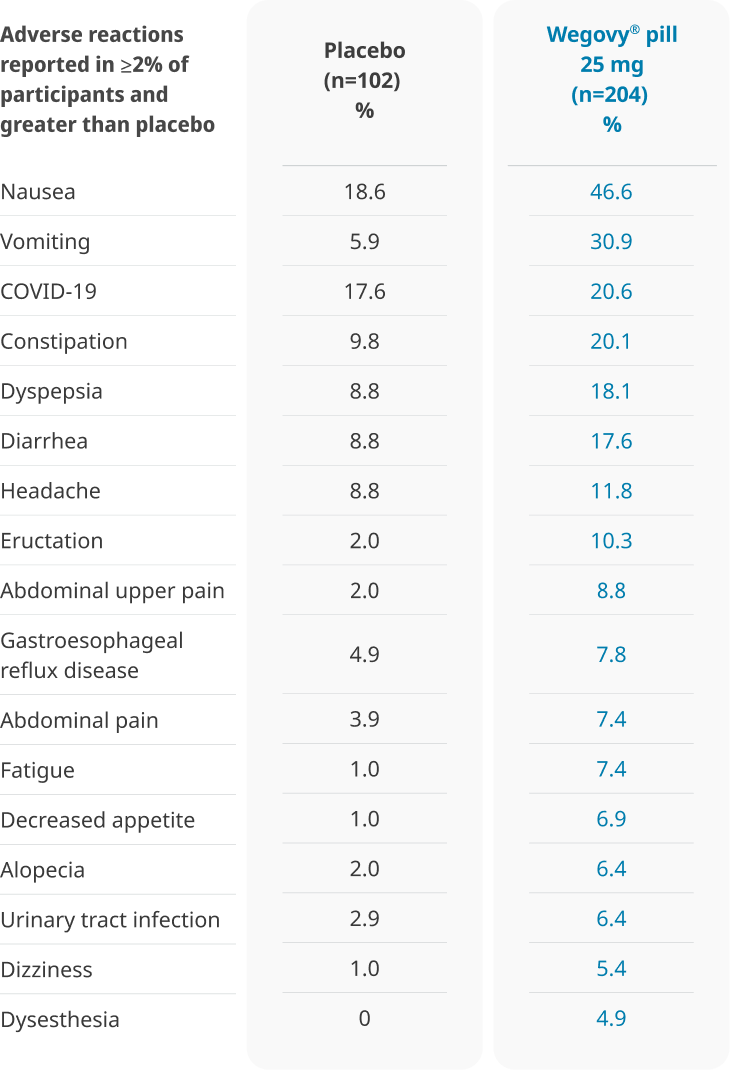
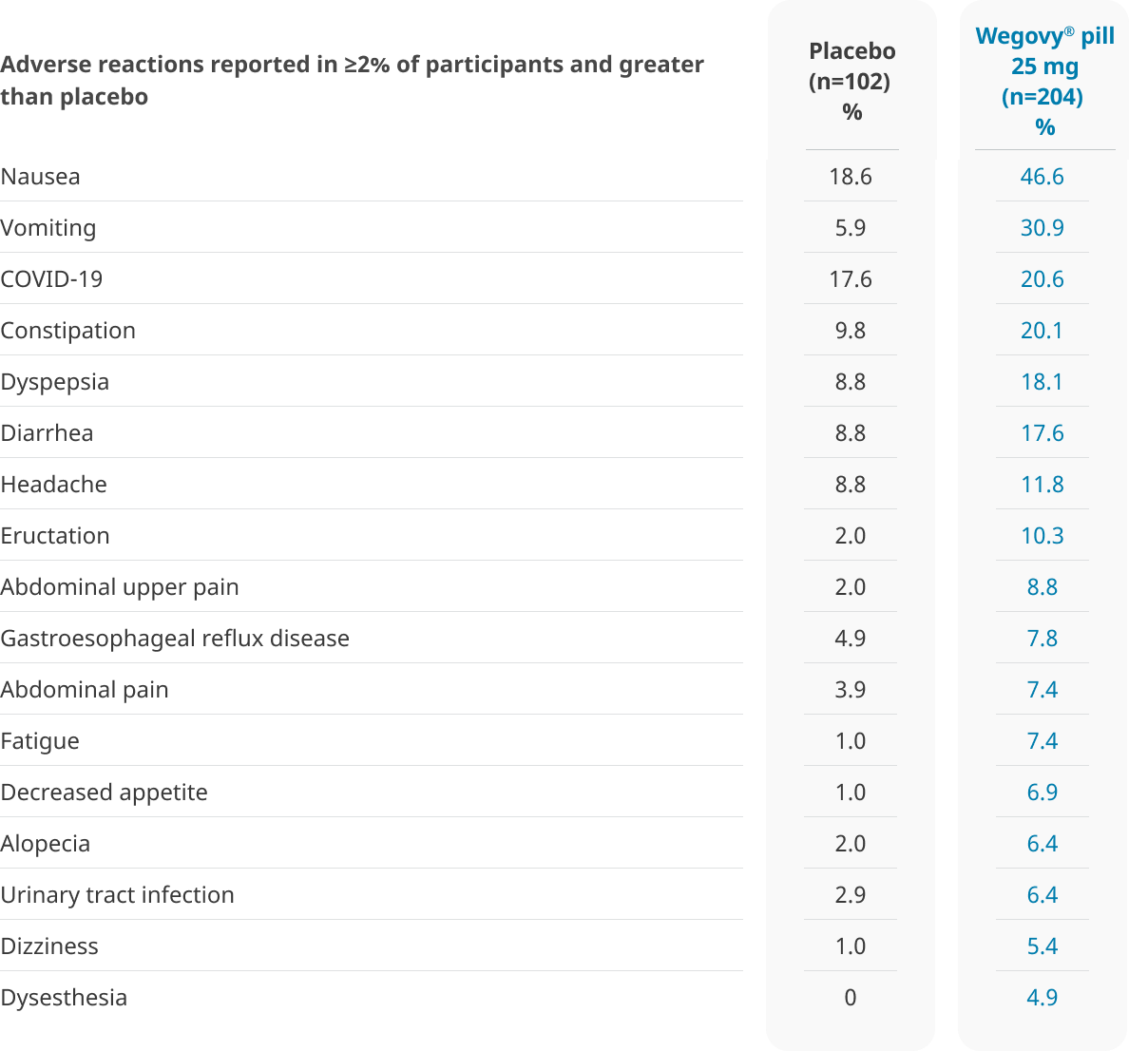
GI, gastrointestinal.
In the 64-week OASIS 4 clinical trial:
- 6.9% of patients treated with once-daily Wegovy® pill 25 mg and 5.9% of patients treated with placebo permanently discontinued treatment as a result of adverse reactions1
- The most common event type leading to discontinuation was gastrointestinal adverse reactions: 3.4% for Wegovy® pill vs 2.0% for placebo1
- The most common adverse reactions leading to discontinuation were: Nausea (1.5% vs 0%), sleep disorder (1.0% vs 0%), abdominal pain (0.5% vs 0%), dyspepsia (0.5% vs 0%), and vomiting (0.5% vs 0%) for the Wegovy® pill and placebo, respectively1
GI adverse reactions were generally transient and most prevalent during the dose-escalation period3
- Median duration of nausea: 13 days for patients on the Wegovy® pill vs 12 days for patients on placebo
- Median duration of vomiting: 2 days for patients on the Wegovy® pill vs 1 day for patients on placebo
- Median duration of diarrhea: 4 days for patients on the Wegovy® pill vs 4 days for patients on placebo
- Median duration of constipation: 26 days for patients on the Wegovy® pill vs 127 days for patients on placebo
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those less than 12 years of age.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients.
Learn about dose escalation with Wegovy®
Get your patients started on Wegovy®
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea, or vomiting. If pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® injection-treated pediatric patients aged ≥12 years than in Wegovy® injection-treated adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® injection-treated patients and 0.7% of placebo treated patients, and by 2.5% of Wegovy® tablet-treated patients and 1% of placebo treated patients. Cholecystitis was reported by 0.6% of Wegovy® injection-treated adult patients and 0.2% of placebo treated patients. In a clinical trial in pediatric patients aged ≥12 years, cholelithiasis was reported by 3.8% of Wegovy® injection-treated patients and 0% placebo treated patients. Cholecystitis was reported by 0.8% of Wegovy® injection-treated pediatric patients and 0% placebo treated patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of Wegovy® injection in adult patients with type 2 diabetes (T2D) and a BMI ≥27 kg/m2, hypoglycemia was reported in more patients treated with Wegovy® versus placebo. In glycemic control clinical trials, the risk of hypoglycemia was increased when semaglutide injection or tablet was used concomitantly with insulin or an insulin secretagogue (e.g., sulfonylurea). Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Wegovy® that could lead to volume depletion, especially during initiation and escalation of Wegovy®
- Severe Gastrointestinal (GI) Adverse Reactions: Use of Wegovy® has been associated with GI adverse reactions, sometimes severe. In adult clinical trials, severe GI adverse reactions were reported more frequently among patients receiving Wegovy® than placebo. Severe GI adverse reactions were reported in 4.1% and 0.9% of Wegovy®-injection treated and placebo treated patients, respectively, and in 2% of Wegovy® tablet-treated and 0% of placebo treated patients, respectively. Severe GI adverse reactions have also been reported postmarketing with GLP-1 receptor agonists. Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with T2D: In a trial of adult patients with T2D and BMI ≥27 kg/m2, diabetic retinopathy was reported by 4% of Wegovy® injection-treated patients and 2.7% of placebo patients. In a glycemic control trial evaluating a dose comparable to the 9 mg dose and the 25 mg semaglutide tablet doses in patients with T2D, 1.3% and 1.9% of patients in the 9 mg and 25 mg semaglutide group, respectively, reported moderate-severe non-proliferative diabetic retinopathy events, and 0% and 0.4% reported proliferative retinopathy events, respectively. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® injection-treated adult patients compared to placebo in clinical trials. More adults treated with Wegovy® injection compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% vs 34%) and 20 bpm or more (26% vs 16%). In a clinical trial in pediatric patients aged ≥12 years with normal baseline heart rate, more patients treated with Wegovy® injection compared to placebo had maximum changes in heart rate of 20 bpm or more (54% vs 39%). Findings were similar in a trial with the Wegovy® tablets. Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with T2D, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues or insulin to reduce the risk of hypoglycemia. The addition of Wegovy® in patients treated with insulin has not been evaluated
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®. Consider increased clinical or laboratory monitoring for medications that have a narrow therapeutic index or that require clinical monitoring
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Lactation: A clinical lactation study reported semaglutide concentrations below the lower limit of quantification in human breast milk. However, salcaprozate sodium (SNAC) and/or its metabolites are present in human milk. Because of the unknown potential for serious adverse reactions in the breastfed infant due to the possible accumulation of SNAC, an absorption enhancer for Wegovy® tablets, and because there are alternative formulations of semaglutide that do not contain SNAC that can be used during lactation, advise patients that breastfeeding is not recommended during treatment with Wegovy® tablets
- Pediatric: Adverse reactions with Wegovy® injection-treated pediatric patients ≥12 years with obesity were similar to those reported in adults. Pediatric patients ≥12 years treated with Wegovy® injection had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with T2D treated with Wegovy® injection for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® injection treatment similar to that reported in adults.
The safety and effectiveness of Wegovy® injection have not been established in pediatric patients to reduce the risk of major adverse CV events or to reduce excess body weight and maintain weight reduction long term in those <12 years.
The safety and effectiveness of Wegovy® tablets have not been established in pediatric patients - Geriatric: In the CV outcomes trial, patients ≥75 years reported more hip and pelvis fractures on Wegovy® injection than placebo. Patients ≥75 years (Wegovy® injection and placebo) reported more serious adverse reactions overall compared to younger adult patients
- Type 2 Diabetes: Wegovy® tablets have not been studied for weight reduction in adults with T2D and obesity or overweight. Administration of Wegovy® injection resulted in less weight reduction in patients with T2D and obesity or overweight compared to those without T2D and obesity or overweight
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg and Wegovy® (semaglutide) tablets 25 mg are indicated in combination with a reduced calorie diet and increased physical activity to:
- Reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight
- Reduce excess body weight and maintain weight reduction long term in adults with obesity or overweight in the presence of at least one weight-related comorbidity
Wegovy® (semaglutide) injection 1.7 mg or 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 and older with obesity
Limitations of Use:
Concomitant use of Wegovy® tablets or Wegovy® injection with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
References
- Wegovy® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
- Data on file. Novo Nordisk Inc.; Plainsboro, NJ.
- Wharton S, Lingvay I, Bogdanski P, et al. Oral semaglutide at a dose of 25 mg in adults with overweight or obesity. N Engl J Med. 2025;393(11):1077-1087.
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232.
- Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245-2257.