Talking to patients about their Fiasp® prescription

What is Fiasp®?
Fiasp® is a rapid-acting human insulin analog indicated to improve glycemic control in adults and children with diabetes mellitus.
Fiasp® is fast
Onset of appearance of ~2.5 minutes1
Fiasp® is familiar
A formulation of insulin aspart with 2 excipients added1,2
Available in FlexTouch®, PenFill®, and vial
Dose timing is different for Fiasp® vs NovoLog® (insulin aspart) injection 100 U/mL1,3
NovoLog®

Fiasp®
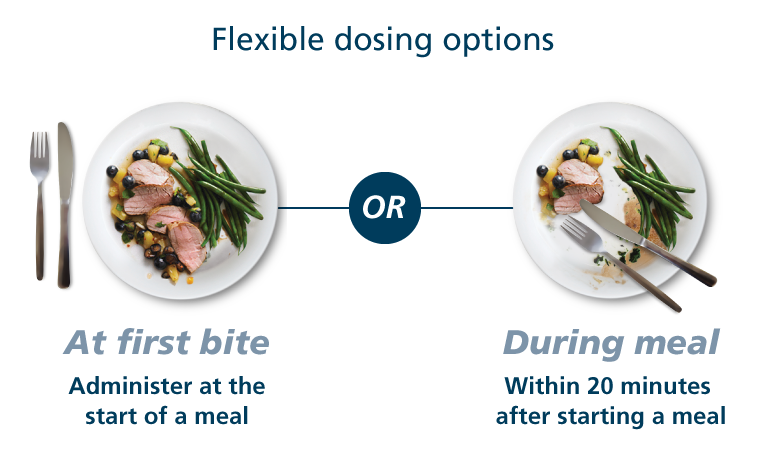
Flexible dosing options
At first bite
Administer at the start of a meal

During meal
Within 20 minutes after starting a meal
Instruct patients on basal-bolus treatment who forget a mealtime dose to monitor their blood glucose level to decide if an insulin dose is needed and to resume their usual dosing schedule at the next meal.
See how dosing options can help patients at mealtime
Fiasp® Trade Unit Product Information

Fiasp® and NovoLog® have the same generic name, insulin aspart 100 U/mL, so be sure to confirm the brand name if it isn’t specified on the written script or in the ePrescribing system.
3 mL single-patient-use Fiasp® FlexTouch® pen
NDC number
0169-3204-15
Form/strength
100 units/mL
Pens per package
5
Units of insulin per pen
300
Units of insulin per package
1500
Max dose per injection
80
Dose increment
1 unit

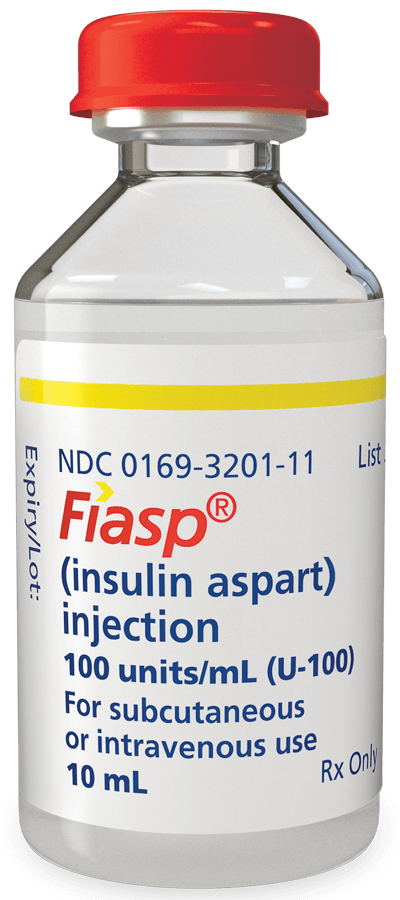
10 mL multiple-dose vials
NDC number
0169-3201-11
Form/strength
100 units/mL
PenFill® cartridge
NDC number
0169-3205-15
Form/strength
100 units/mL
Cartridges per package
5
Units per cartridge
300
Units per package
1500

PumpCart® cartridge
NDC number
0169-3206-15
Form/strength
100 units/mL
Cartridges per package
5
Units per cartridge
160
Units per package
800

Help patients save
Eligible commercially insured patients can save on prescriptions of Fiasp® with the savings offer. Direct patients to FiaspSavings.com to get the offer.

Filling a basal insulin script?
Find out what you need to know about this basal insulin option from Novo Nordisk.
See how Fiasp® FlexTouch® works with the Instructions For Use
Show patients the illustrated step-by-step Instructions For Use. Patients can learn more about Fiasp® by viewing this video on MyFiasp.com.
See the features of FlexTouch®, the latest pen technology from Novo Nordisk.
How to store Fiasp®
Fiasp® FlexTouch®
Not in use (unopened): If unopened, Fiasp® FlexTouch® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® FlexTouch® can be stored at room temperature (below 86°F [30°C]) or in the refrigerator (36°F to 46°F [2°C to 8°C]) without the needle attached for a maximum of 4 weeks (28 days).1
Fiasp® vial
Not in use (unopened): If unopened, Fiasp® vials should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® vials can be stored at room temperature (below 86°F [30°C]) or in the refrigerator (36°F to 46°F [2°C to 8°C]) for a maximum of 4 weeks (28 days).1
Fiasp® PenFill®
Not in use (unopened): If unopened, Fiasp® PenFill® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® PenFill® can be stored at room temperature (below 86°F [30°C]) for a maximum of 4 weeks (28 days).1 Do not refrigerate Fiasp® PenFill® cartridges after first use.
Fiasp® PumpCart®
Not in use (unopened): If unopened, Fiasp® PumpCart® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 18 days.1
In use (opened): After first use, Fiasp® PumpCart® can be kept below 98.6°F (37°C) for up to 4 days. The maximum time at room temperature is 18 days, including 4 days in the pump.1 Do not refrigerate Fiasp® PumpCart® cartridges after first use.
Support for patients
NovoCare® offers bite-sized, everyday lessons on eating better, moving more, and treating diabetes that your patients can learn in 5 minutes.
Important Safety Information for Fiasp®
Contraindications
- Fiasp® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Fiasp® or one of its excipients.
Warnings and Precautions
- Never share a Fiasp® FlexTouch® Pen, PenFill® cartridge or PenFill® cartridge device between patients, even if the needle is changed. Patients using Fiasp® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia is the most common adverse reaction of insulin, including Fiasp®, and may be life-threatening. Increase glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness.
- To avoid medication errors and accidental mix-ups between Fiasp® and other insulin products, instruct patients to always check the insulin label before injection.
- As with all insulins, Fiasp® use can lead to life-threatening hypokalemia, which then may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including Fiasp®.
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Fiasp®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of Fiasp® may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Drug Interactions
- Drugs That May Increase the Risk of Hypoglycemia: Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose reductions and increased frequency of glucose monitoring may be required.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Fiasp®: Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. Dose increases and increased frequency of glucose monitoring may be required.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Fiasp®: Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of glucose monitoring may be required
Adverse Reactions
- Adverse reactions observed with Fiasp® include hypoglycemia, allergic reactions, hypersensitivity, injection site reactions, lipodystrophy, and weight gain.
Use in Specific Populations
- Pediatric patients with type 1 diabetes treated with mealtime and postmeal Fiasp® reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog® (insulin aspart) injection; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients.
- Like all insulins, Fiasp® requirements may be reduced in patients with renal impairment or hepatic impairment. These patients may require more frequent blood glucose monitoring and dose adjustments.
Please click here for Fiasp® Prescribing Information.
Important Safety Information for NovoLog®
Contraindications
- NovoLog® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to NovoLog® or one of its excipients.
Warnings and Precautions
- Never Share a NovoLog® FlexPen, NovoLog® FlexTouch®, PenFill® Cartridge, or PenFill® Cartridge Device Between Patients, even if the needle is changed. Patients using NovoLog® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse effect of all insulins, including NovoLog®. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers), or in patients who experience recurrent hypoglycemia.
- Risk Factors for Hypoglycemia: The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of NovoLog® may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to co-administered medication. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia.
- Hypoglycemia Due to Medication Errors: To avoid medication errors and accidental mix-ups between NovoLog® and other insulin products, instruct patients to always check the insulin label before injection.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including NovoLog®.
- Hypokalemia: All insulins, including NovoLog®, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentration).
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including NovoLog®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Malfunction of the insulin pump or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Patients using insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
NovoLog® continuous subcutaneous infusion route (insulin pump): Do not mix NovoLog® with any other insulin or diluent.
Adverse Reactions
- Adverse reactions observed with NovoLog® include hypoglycemia, allergic reactions, local injection site reactions, lipodystrophy, rash, and pruritus.
Drug Interactions
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine.
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine.
Please click here for NovoLog® Prescribing Information.
Important Safety Information for Fiasp®
Contraindications
- Fiasp® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Fiasp® or one of its excipients.
Warnings and Precautions
- Never share a Fiasp® FlexTouch® Pen, PenFill® cartridge or PenFill® cartridge device between patients, even if the needle is changed. Patients using Fiasp® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia is the most common adverse reaction of insulin, including Fiasp®, and may be life-threatening. Increase glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness.
- To avoid medication errors and accidental mix-ups between Fiasp® and other insulin products, instruct patients to always check the insulin label before injection.
- As with all insulins, Fiasp® use can lead to life-threatening hypokalemia, which then may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including Fiasp®.
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Fiasp®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of Fiasp® may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Drug Interactions
- Drugs That May Increase the Risk of Hypoglycemia: Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose reductions and increased frequency of glucose monitoring may be required.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Fiasp®: Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. Dose increases and increased frequency of glucose monitoring may be required.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Fiasp®: Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of glucose monitoring may be required
Adverse Reactions
- Adverse reactions observed with Fiasp® include hypoglycemia, allergic reactions, hypersensitivity, injection site reactions, lipodystrophy, and weight gain.
Use in Specific Populations
- Pediatric patients with type 1 diabetes treated with mealtime and postmeal Fiasp® reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog® (insulin aspart) injection; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients.
- Like all insulins, Fiasp® requirements may be reduced in patients with renal impairment or hepatic impairment. These patients may require more frequent blood glucose monitoring and dose adjustments.
Please click here for Fiasp® Prescribing Information.
Important Safety Information for Fiasp®
Contraindications
- Fiasp® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Fiasp® or one of its excipients.
Warnings and Precautions
- Never share a Fiasp® FlexTouch® Pen, PenFill® cartridge or PenFill® cartridge device between patients, even if the needle is changed. Patients using Fiasp® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia is the most common adverse reaction of insulin, including Fiasp®, and may be life-threatening. Increase glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness.
- To avoid medication errors and accidental mix-ups between Fiasp® and other insulin products, instruct patients to always check the insulin label before injection.
- As with all insulins, Fiasp® use can lead to life-threatening hypokalemia, which then may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including Fiasp®.
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Fiasp®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of Fiasp® may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Drug Interactions
- Drugs That May Increase the Risk of Hypoglycemia: Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose reductions and increased frequency of glucose monitoring may be required.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Fiasp®: Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. Dose increases and increased frequency of glucose monitoring may be required.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Fiasp®: Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of glucose monitoring may be required
Adverse Reactions
- Adverse reactions observed with Fiasp® include hypoglycemia, allergic reactions, hypersensitivity, injection site reactions, lipodystrophy, and weight gain.
Use in Specific Populations
- Pediatric patients with type 1 diabetes treated with mealtime and postmeal Fiasp® reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog® (insulin aspart) injection; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients.
- Like all insulins, Fiasp® requirements may be reduced in patients with renal impairment or hepatic impairment. These patients may require more frequent blood glucose monitoring and dose adjustments.
Please click here for Fiasp® Prescribing Information.
Important Safety Information for NovoLog®
Contraindications
- NovoLog® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to NovoLog® or one of its excipients.
Warnings and Precautions
- Never Share a NovoLog® FlexPen, NovoLog® FlexTouch®, PenFill® Cartridge, or PenFill® Cartridge Device Between Patients, even if the needle is changed. Patients using NovoLog® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse effect of all insulins, including NovoLog®. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers), or in patients who experience recurrent hypoglycemia.
- Risk Factors for Hypoglycemia: The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of NovoLog® may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to co-administered medication. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia.
- Hypoglycemia Due to Medication Errors: To avoid medication errors and accidental mix-ups between NovoLog® and other insulin products, instruct patients to always check the insulin label before injection.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including NovoLog®.
- Hypokalemia: All insulins, including NovoLog®, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentration).
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including NovoLog®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Malfunction of the insulin pump or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Patients using insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
NovoLog® continuous subcutaneous infusion route (insulin pump): Do not mix NovoLog® with any other insulin or diluent.
Adverse Reactions
- Adverse reactions observed with NovoLog® include hypoglycemia, allergic reactions, local injection site reactions, lipodystrophy, rash, and pruritus.
Drug Interactions
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine.
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine.
Please click here for NovoLog® Prescribing Information.
References:
- Fiasp [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56(5):551-559.
- NovoLog [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
