Fiasp® can be administered in a variety of ways
Prefilled pen, durable pen with a cartridge, vial and syringe, an insulin pump, or automated insulin delivery system with cartridge
Refer to the insulin pump or automated insulin delivery system user manual to see if Fiasp® can be used. Use in accordance with the insulin pump or automated insulin delivery system instructions for use.
The Fiasp® FlexTouch® Pen

No push-button extension
No matter what dose is dialed, the Fiasp® FlexTouch® push button doesn’t extend
Dosing from 1-80 units2,3
Patients may hear or feel a click when the FlexTouch® dose is delivereda,b
aAfter the dose display has returned to 0, the needle should be kept under the skin for at least 6 seconds to ensure the full dose is delivered. Refer to complete Instructions For Use on how to properly administer your patients’ insulin.
bUse Fiasp® FlexTouch® pen with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
Fiasp® FlexTouch® pen instructions
Follow the illustrated step-by-step Instructions For Use for Fiasp® FlexTouch®. Patients can view this video on MyFiasp.com
Novo Nordisk offers patient support, including resources detailing how to administer Fiasp®
10-mL vial
For administration by syringe, by IV infusion, or through an insulin pump
Allows for half-unit dosing1

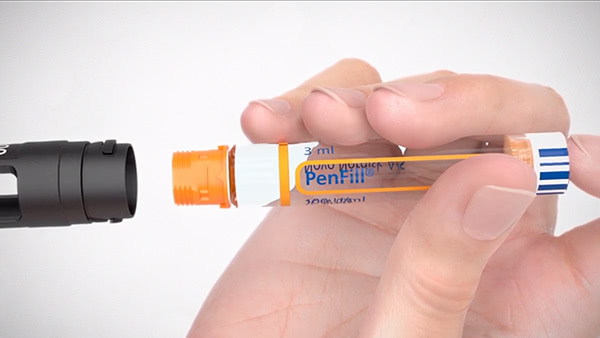
PenFill® cartridge
Designed for use with durable insulin delivery devices, such as NovoPen Echo®
Allows for half-unit dosing4
Lower environmental impact compared to prefilled disposable insulin pens
Fiasp® PenFill® is approved for use with NovoPen Echo®


Durable pen that lasts up to 5 years
More precise half-unit dosing
Automatically records the last dose and approximate time since the last injection4
A separate prescription is required for NovoPen Echo®, Fiasp® PenFill®, and needles. Needles are sold separately and need to be processed by a pharmacist.
NovoPen Echo® instructions
Follow the illustrated step-by-step Instructions For Use for NovoPen Echo®. Patients can view this video on MyFiasp.com
Patients with commercial insurance may be eligible to pay as little as $0 for their NovoPen Echo® with a savings card.e Direct patients to NovoPenEchoSavings.com to get their savings card.

PumpCart® cartridge
- 1.6 mL single-patient-use PumpCart® cartridges are ready for use directly in compatible insulin pumps1
- Check the insulin pump manual to see if PumpCart® can be used with your patient's pump
PumpCart® should be used in accordance with the insulin pump’s instructions for use.
iLet Bionic Pancreas is cleared for use with Fiasp® Pumpcart®
The iLet Bionic Pancreas is designed to use rapid-acting U-100 insulin. The Fiasp® PumpCart® pre-filled 1.6 mL cartridge has been tested and is able to be used in the iLet Bionic Pancreas for people with type 1 diabetes 6 years of age and older.5
Fiasp® is compatible with the system for use up to 72 hours (3 days).
How to store Fiasp®
Fiasp® FlexTouch®
Not in use (unopened): If unopened, Fiasp® FlexTouch® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® FlexTouch® can be stored at room temperature (below 86°F [30°C]) or in the refrigerator (36°F to 46°F [2°C to 8°C]) without the needle attached for a maximum of 4 weeks (28 days).1
Fiasp® vial
Not in use (unopened): If unopened, Fiasp® vials should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® vials can be stored at room temperature (below 86°F [30°C]) or in the refrigerator (36°F to 46°F [2°C to 8°C]) for a maximum of 4 weeks (28 days).1
Fiasp® PenFill®
Not in use (unopened): If unopened, Fiasp® PenFill® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 4 weeks.1
In use (opened): After first use, Fiasp® PenFill® can be stored at room temperature (below 86°F [30°C]) for a maximum of 4 weeks (28 days).1 Do not refrigerate Fiasp® PenFill® cartridges after first use.
Fiasp® PumpCart®
Not in use (unopened): If unopened, Fiasp® PumpCart® should be stored in a refrigerator (36°F to 46°F [2°C to 8°C]) until expiration date. If unopened but stored outside of refrigerated conditions up to 86°F, begin in-use storage for a maximum of 18 days.1
In use (opened): After first use, Fiasp® PumpCart® can be kept below 98.6°F (37°C) for up to 4 days. The maximum time at room temperature is 18 days, including 4 days in the pump.1 Do not refrigerate Fiasp® PumpCart® cartridges after first use.
Select Novo Nordisk samples are available
Important Safety Information for Fiasp®
Contraindications
- Fiasp® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Fiasp® or one of its excipients.
Warnings and Precautions
- Never share a Fiasp® FlexTouch® Pen, PenFill® cartridge or PenFill® cartridge device between patients, even if the needle is changed. Patients using Fiasp® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia is the most common adverse reaction of insulin, including Fiasp®, and may be life-threatening. Increase glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness.
- To avoid medication errors and accidental mix-ups between Fiasp® and other insulin products, instruct patients to always check the insulin label before injection.
- As with all insulins, Fiasp® use can lead to life-threatening hypokalemia, which then may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including Fiasp®.
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Fiasp®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of Fiasp® may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Drug Interactions
- Drugs That May Increase the Risk of Hypoglycemia: Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose reductions and increased frequency of glucose monitoring may be required.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Fiasp®: Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. Dose increases and increased frequency of glucose monitoring may be required.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Fiasp®: Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of glucose monitoring may be required
Adverse Reactions
- Adverse reactions observed with Fiasp® include hypoglycemia, allergic reactions, hypersensitivity, injection site reactions, lipodystrophy, and weight gain.
Use in Specific Populations
- Pediatric patients with type 1 diabetes treated with mealtime and postmeal Fiasp® reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog® (insulin aspart) injection; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients.
- Like all insulins, Fiasp® requirements may be reduced in patients with renal impairment or hepatic impairment. These patients may require more frequent blood glucose monitoring and dose adjustments.
Please click here for Fiasp® Prescribing Information.
Important Safety Information for Fiasp®
Contraindications
- Fiasp® is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Fiasp® or one of its excipients.
Warnings and Precautions
- Never share a Fiasp® FlexTouch® Pen, PenFill® cartridge or PenFill® cartridge device between patients, even if the needle is changed. Patients using Fiasp® vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia is the most common adverse reaction of insulin, including Fiasp®, and may be life-threatening. Increase glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness.
- To avoid medication errors and accidental mix-ups between Fiasp® and other insulin products, instruct patients to always check the insulin label before injection.
- As with all insulins, Fiasp® use can lead to life-threatening hypokalemia, which then may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated.
- Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulin products, including Fiasp®.
- Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including Fiasp®. Patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of the TZD must be considered.
- Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of Fiasp® may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Drug Interactions
- Drugs That May Increase the Risk of Hypoglycemia: Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose reductions and increased frequency of glucose monitoring may be required.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Fiasp®: Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. Dose increases and increased frequency of glucose monitoring may be required.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Fiasp®: Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia: Beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of glucose monitoring may be required
Adverse Reactions
- Adverse reactions observed with Fiasp® include hypoglycemia, allergic reactions, hypersensitivity, injection site reactions, lipodystrophy, and weight gain.
Use in Specific Populations
- Pediatric patients with type 1 diabetes treated with mealtime and postmeal Fiasp® reported a higher rate of blood glucose confirmed hypoglycemic episodes compared to patients treated with NovoLog® (insulin aspart) injection; the imbalance was greater during the nocturnal period. Monitor blood glucose levels closely in pediatric patients.
- Like all insulins, Fiasp® requirements may be reduced in patients with renal impairment or hepatic impairment. These patients may require more frequent blood glucose monitoring and dose adjustments.
Please click here for Fiasp® Prescribing Information.
iLet Bionic Pancreas System Important Safety Information
The iLet Bionic Pancreas System is indicated for use by people with type 1 diabetes 6 years of age and older. The iLet Bionic Pancreas requires prescription by a physician. Refer to the iLet Bionic Pancreas System User Guide at www.betabionics.com/resouces/user-guides, or for complete safety information including indications, contraindications, warnings, cautions, compatible devices, compatible drugs and instructions, refer to www.betabionics.com/resources/safety. DO NOT start to use the iLet Bionic Pancreas System without adequate training. Incorrect use may result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia.
References:
- Fiasp [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Wielandt JO, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. An assessment of dose accuracy and injection force of a novel prefilled insulin pen: comparison with a widely used prefilled insulin pen. Expert Opin Drug Deliv. 2011;8(10):1271-1276.
- Wielandt JO, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. FlexTouch: a prefilled insulin pen with a novel injection mechanism with consistent high accuracy at low- (1 U), medium- (40 U), and high- (80 U) dose settings. J Diabetes Sci Technol. 2011;5(5):1195-1199.
- NovoPen Echo. User guide. Plainsboro, NJ: Novo Nordisk Inc.
- iLet Bionic Pancreas System. User Guide. Irvine, CA; 2023.