For on-demand bleed control, surgery, and routine prophylaxis in adults and children with hemophilia A.
Novoeight® dosing guidelines
Novoeight® is available as a white lyophilized powder in single-use vials of six different dose strengths.1
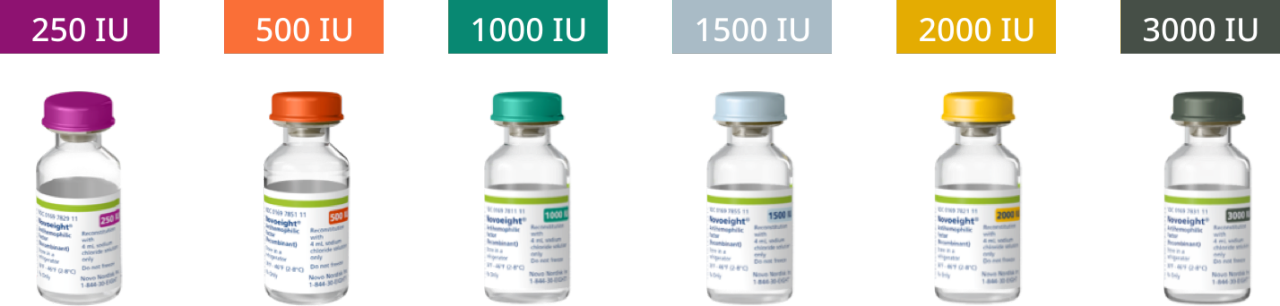

Calculate dosage for your patients1
Refer to dosing tables below for additional guidance.
For complete dosing information, please see the Prescribing Information.
Dosage and duration of treatment depend on the severity of the factor VIII deficiency, the location and extent of bleeding, and the patient’s clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
Each vial of Novoeight® contains the labeled amount of recombinant factor VIII in international units (IU). One IU of factor VIII activity corresponds to the quantity of factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of factor VIII is based on the empirical finding that one IU of factor VIII per kg body weight raises the plasma factor VIII activity by two IU/dL. This relationship causes a factor of 0.5 to be present in the dose calculation formula shown above.
Base the dose and frequency of Novoeight® on the individual clinical response. Patients may vary in their pharmacokinetic and clinical responses.
Novoeight® dosing by clinical condition
Control and prevention of bleeding episodes1
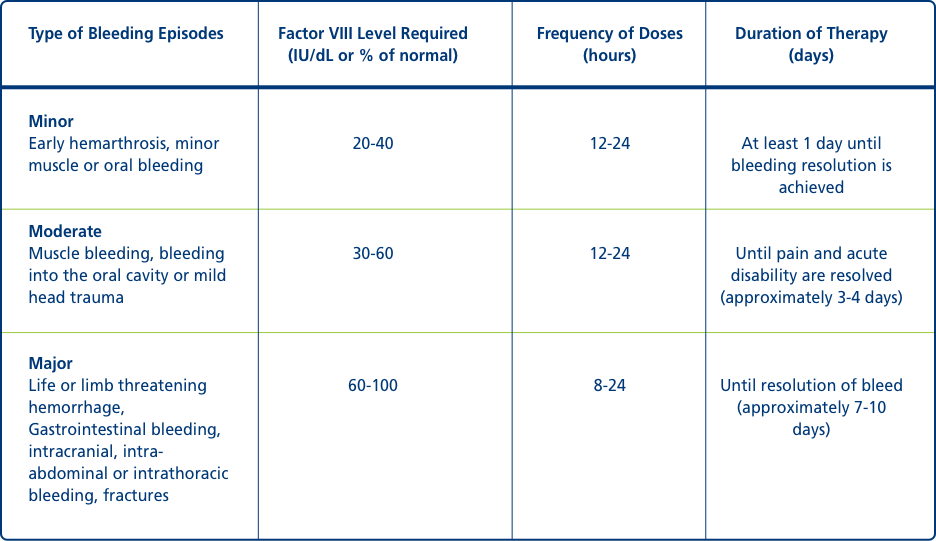
Type of Bleeding Episodes
Minor
Early hemarthrosis, minor muscle or oral bleeding
Factor VIII Level Required (IU/dL or % of normal): 20-40
Frequency of Doses (hours): 12-24
Duration of Therapy (days): At least 1 day until bleeding resolution is achieved
Moderate
Muscle bleeding, bleeding into the oral cavity or mild head trauma
Factor VIII Level Required (IU/dL or % of normal): 30-60
Frequency of Doses (hours): 12-24
Duration of Therapy (days): Until pain and acute disability are resolved (approximately 3-4 days)
Major
Life or limb threatening hemorrhage, Gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, fractures
Factor VIII Level Required (IU/dL or % of normal): 60-100
Frequency of Doses (hours): 8-24
Duration of Therapy (days): Until resolution of bleed (approximately 7-10 days)
Perioperative management1
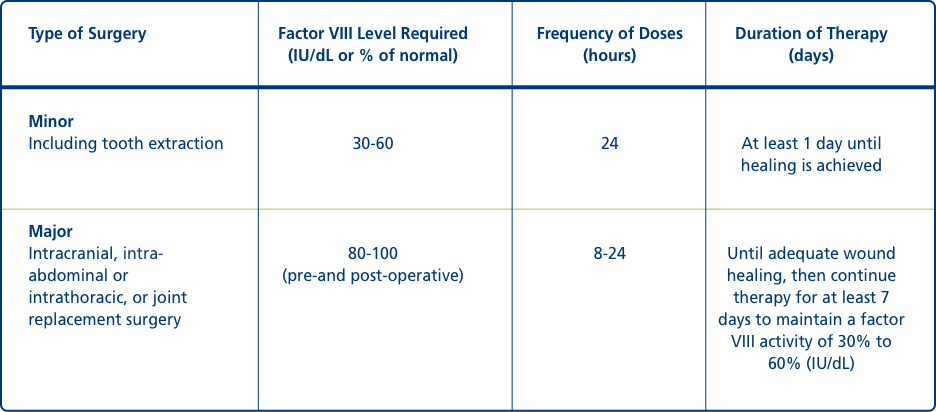
Type of Surgery
Minor
Including tooth extraction
Factor VIII Level Required (IU/dL or % of normal): 30-60
Frequency of Doses (hours): 24
Duration of Therapy (days): At least 1 day until healing is achieved
Major
Intracranial, intra-abdominal or intrathoracic or joint replacement surgery
Factor VIII Level Required (IU/dL or % of normal): 80-100 (pre-and postoperative)
Frequency of Doses (hours): 8-24
Duration of Therapy (days): Until adequate wound healing, then continue therapy for at least 7 days to maintain a factor VIII activity of 30% to 60% (IU/dL)
Routine prophylaxis1
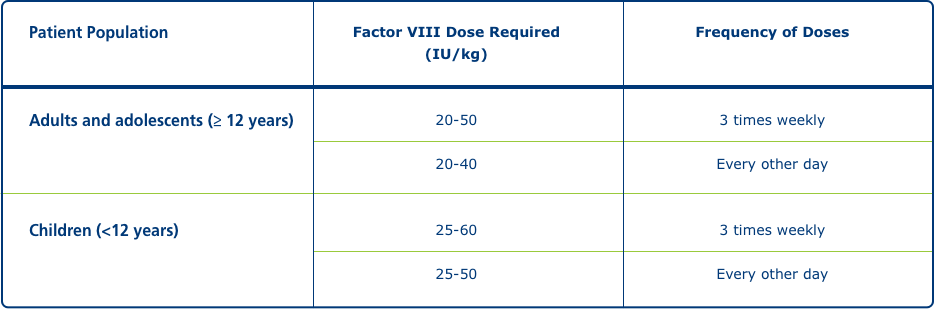
Patient Population
Adults and adolescents (≥ 12 years)
Factor VIII Dose Required (IU/kg): 20-50
Frequency of Doses: 3 times weekly;
Factor VIII Dose Required (IU/kg): 20-40
Frequency of Doses: Every other day
Children (<12 years)
Factor VIII Dose Required (IU/kg): 25-60
Frequency of Doses: 3 times weekly;
Factor VIII Dose Required (IU/kg): 25-50
Frequency of Doses: Every other day
Reconstituting a dose of Novoeight® is as easy as attach, twist, and mix1
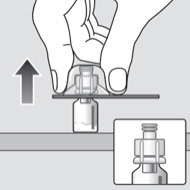
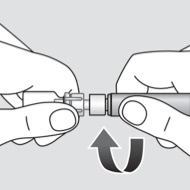
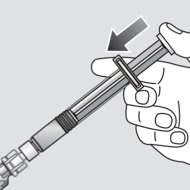
Administration instructions for Novoeight®1
For intravenous injection only.
- Accidental needle stick with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis.
Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single-use - Inspect the reconstituted Novoeight® solution visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed
- Do not administer Novoeight® in the same tubing or container with other medicinal products
Administer Novoeight® using the following procedures:
- Invert the Novoeight® vial and slowly draw the solution into the syringe
- Detach the syringe from the vial adapter by turning the syringe counterclockwise
- Attach the syringe to the Luer end of an infusion needle set
- Inject the reconstituted Novoeight® intravenously slowly over 2 to 5 minutes
- After injection, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused Novoeight® and other waste materials
Please see the Prescribing Information for further details.
Caution:
- The prefilled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector
- Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, InVision-Plus® Junior®, Bionector®), and their use can damage the connector and affect administration. To administer product through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe
- If you have encountered any problems with attaching the prefilled histidine diluent syringe to any Luer-lock compatible device, please contact Novo Nordisk at (844) 303-4448
Try an rFVIII with one proven dose

Please click here for Esperoct® Prescribing Information and see Important Safety Information at the bottom of the page.
ABR=annualized bleed rate.
aRegimen can be individually tailored to less or more frequent dosing based on bleeding episodes.2
bFor minor surgeries, additional dose(s) can be administered after 24 hours; for major surgeries, additional doses can be administered every 24 hours in the first week and then approximately every 48 hours until wound healing has occurred.2
cAdditional dose can be administered every 24 hours for major or life-threatening bleeding.2
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Esperoct®
Contraindications
- Do not use in patients who have known hypersensitivity to Esperoct® or its components, including hamster proteins
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate treatment
- Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay that measures Factor VIII inhibitor concentration if bleeding is not controlled with the recommended dose of Esperoct® or if the expected plasma Factor VIII activity levels are not attained
- Temporary decrease in Factor VIII incremental recovery (IR) has been observed after Esperoct® infusion, within the first 5 exposure days, in previously untreated patients (PUPs) <6 years of age. During the decreased IR period, these subjects may have an increased bleeding tendency. If bleeding is not controlled with the recommended dose of Esperoct® and/or the expected Factor VIII activity levels are not attained and Factor VIII inhibitors are not detected, consider adjusting the dose, dosing frequency, or discontinuing Esperoct®
Adverse Reactions
- The most frequently reported adverse reactions in clinical trials (≥1%) were rash, redness, itching (pruritus), and injection site reactions. Additional frequently reported adverse reactions (≥1%) in PUPs included Factor VIII inhibition and hypersensitivity.
Please click here for Esperoct® Prescribing Information.
Indications and Usage
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes
- Esperoct® is not indicated for the treatment of von Willebrand disease
References
- Novoeight® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Esperoct® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.

