For on-demand bleed control, surgery, and routine prophylaxis in adults and children with hemophilia A.
Financial programs and support for patients
Novo Nordisk provides assistance through NovoCare®, a network to help your patients access co-pay savings, product support, and more.

NovoCare® is designed for your patients
Patients can access:
- Product support programs that help with treatment costs
- Representatives who speak Spanish to better serve more patients
You can help your patients by calling 1-844-668-6732 or have them contact NovoCare® at NovoCare.com
NovoCare® can help with:
QuickCheck™ benefits verification
Verify your patients’ benefits and receive confirmation of coverage. If no coverage is available, names of alternative therapies may be supplied.
Patient Assistance Program (PAP)
See if your patients are eligible to receive their treatment free of charge. No registration or monthly fees required.
Patient Trial Program
Find out if your patients qualify to receive a limited supply of Novo Nordisk’s product for free.
Patient
enrollment form
Download and complete the patient enrollment form to attest your patient’s need for treatment.
Additional resources for patients
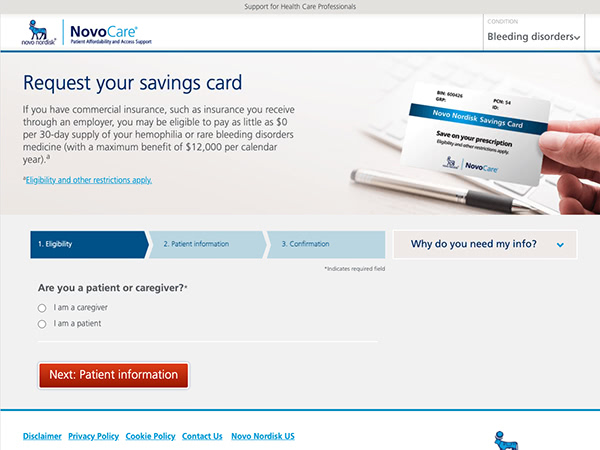
Interim Product Program
This limited program provides hemophilia therapy to qualifying patients during a coverage gap. Contact a NovoCare® Specialist by calling 1-844-668-6732.
Looking for other Novoeight® resources?
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease
Important Safety Information for Novoeight®
Contraindications
- Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight® or its components, including hamster proteins
Warnings and Precautions
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight® and administer appropriate treatment
- Development of activity-neutralizing antibodies (inhibitors) may occur. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight® in PUPs. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors
Adverse Reactions
- The most frequently reported adverse reactions (≥1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia.
Please click here for Novoeight® Prescribing Information.
Indications and Usage
Novoeight® (antihemophilic factor, recombinant) is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management, and routine prophylaxis to reduce the frequency of bleeding episodes.
- Novoeight® is not indicated for the treatment of von Willebrand disease