Routine prophylaxis treatment in a prefilled, subcutaneous pen to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older who have hemophilia B and A with or without inhibitors
Powerful everyday bleed protection for hemophilia B or A with or without inhibitors1‑3,a
aAdherence to daily dosing of Alhemo® is important to maintain protection against bleeding.
Royal lives with hemophilia B with inhibitors,
uses Alhemo®, and is an employee of Novo Nordisk.
Royal lives with hemophilia B with inhibitors,
uses Alhemo®, and is an employee of Novo Nordisk.
Treated spontaneous and traumatic bleeds in patients with HB1,2
Primary Endpoint: The estimated mean ABR ratio in patients with HB was 0.21 (P<0.001)1
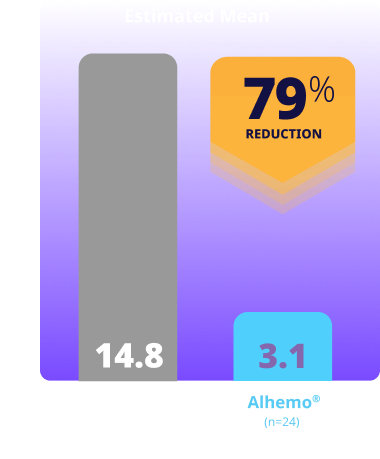
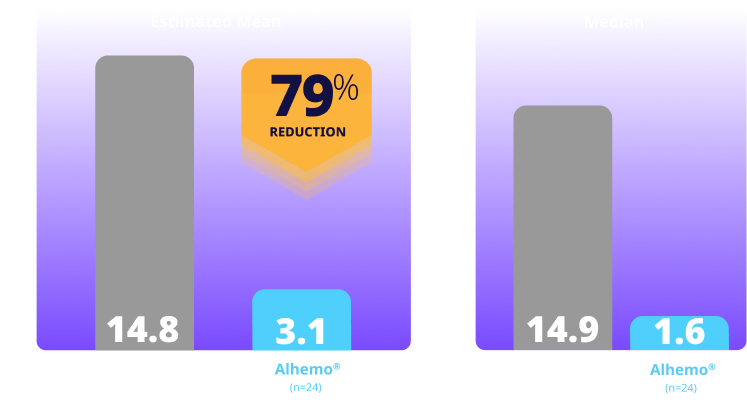
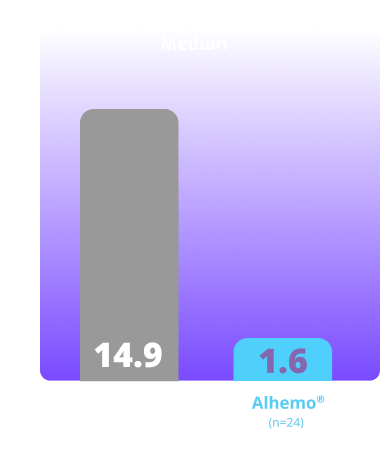
Study design: explorer8 was a phase 3, open-label, study evaluating Alhemo® for routine PPx in 118 adult (67 HA and 51 HB) and 38 adolescent (23 HA and 15 HB) males. Efficacy was evaluated separately in HA and HB when patients previously treated on demand in arms 1 (no PPx; on-demand factor replacement) and 2 (Alhemo® PPx) had completed the main part of the trial (at least 24 or at least 32 weeks, respectively), based on the number of treated bleeding episodes, comparing Alhemo® PPx with on-demand factor. Ratio of the ABR was estimated using a negative binomial model.1
In HA, using a negative binomial model, a ratio of the ABR was estimated to 0.14 (P<0.001), corresponding to a reduction in ABR of 86% for patients on Alhemo® PPx compared with on-demand FVIII. The mean ABR was 2.7 [95% CI: 1.63; 4.59] for patients on Alhemo® PPx (arm 2) and 19.3 [95% CI: 11.25; 33.03] for patients receiving on-demand FVIII (arm 1).1
ABR=annualized bleeding rate; BPA=bypassing agents; HAwI=hemophilia A with inhibitors; HBwI=hemophilia B with inhibitors; IQR=interquartile range; PPx=prophylaxis.
The confirmatory secondary endpoint (non-inferiority analysis) compared data in the intrapatient analysis set, evaluating the number of treated spontaneous and traumatic bleeding episodes while on prior factor PPx (among patients on therapy for ≥24 weeks) in the non-interventional explorer6 study with data while on Alhemo® PPx in the phase 3 explorer8 trial.
The mean ABR ratio was 1.39 (95% CI 0.73-2.63) for HA and 1.75 (0.81-3.78) for HB. Non-inferiority of Alhemo® to previous PPx was not established because the upper limit of the estimated 95% CI for the annualized bleeding rate ratio was greater than the non-inferiority margin of 2.0.
In 22 patients with HB, the mean ABR was 5.4 [95% CI: 2.3; 12.9] for those on Alhemo® vs 3.1 [95% CI: 2.1; 4.6] for those on prior FIX PPx. The median ABR was 1.4 [IQR: 0.0-8.1] for Alhemo® vs 2.1 [IQR: 0.9-4.2] for those on prior FIX PPx.
In 29 patients with HA, the mean ABR was 5.1 [95% CI: 2.7; 9.7] for those on Alhemo® vs 3.7 [95% CI: 2.5; 5.4] for those on prior FVIII PPx. The median ABR was 2.3 [IQR: 0.0-4.7] for Alhemo® vs 2.2 [IQR: 0.8-6.2] for those on prior FVIII PPx.
Additional analysis:
Discover lower target joint bleed rate and more bleed-free patients with HB
63% lower bleed rate in target joints
Supportive secondary end point was an estimated mean target joint ABR of 1.4 (95% CI: 0.7-2.9) with Alhemo® (n=24) vs 3.9 (95% CI: 1.5-10.1) with on-demand FIX (n=12), corresponding to an estimated mean target joint ABR ratio of 0.37.2,a
Not alpha controlled
42% bleed-free for 24 weeks
for patients with HB on Alhemo® (n=24) vs 8% receiving on-demand FIX (n=12)2,b
Additional assessment that is not alpha controlled
Study design: explorer8 was a phase 3, open-label study evaluating Alhemo® for routine PPx in 118 adult (67 HA and 51 HB) and 38 adolescent (23 HA and 15 HB) male patients. Efficacy was evaluated separately in HA and HB when patients previously treated on demand in arms 1 (no PPx; on-demand factor) and 2 (Alhemo® PPx) had completed the main part of the trial (at least 24 or at least 32 weeks, respectively), based on the number of treated bleeding episodes, comparing Alhemo® PPx with on-demand factor. Using a negative binomial model, a ratio of the ABRs was estimated to 0.14 (P<0.001) for patients with hemophilia A and 0.21 (P<0.001) for patients with hemophilia B for Alhemo® PPx compared with on-demand factor.2
Treated spontaneous and traumatic bleeds1,3
Primary Endpoint: The estimated mean ABR ratio was 0.14 (P<0.001)1
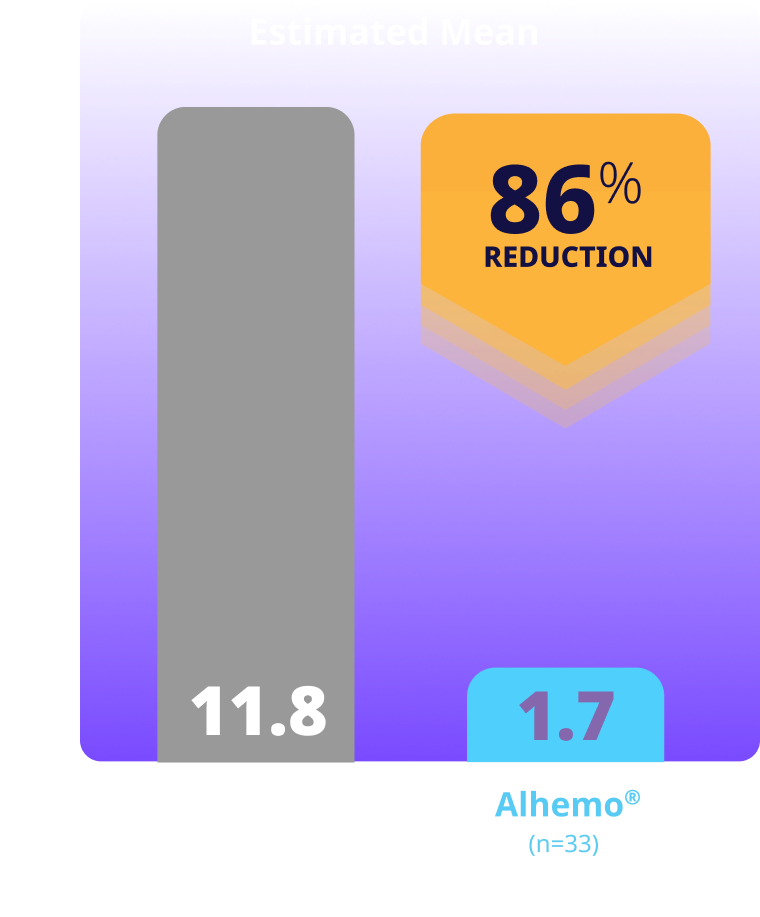
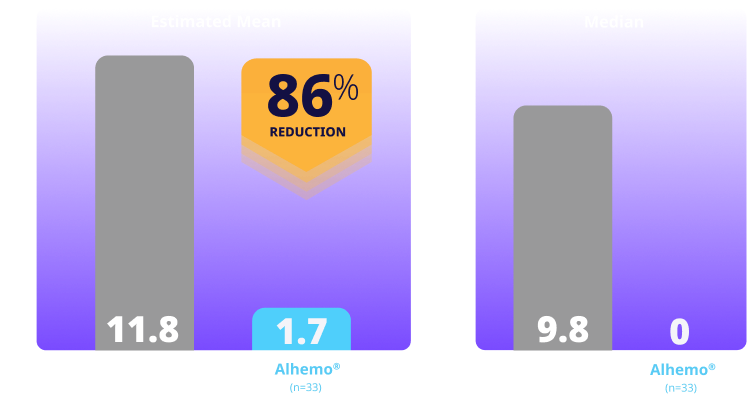
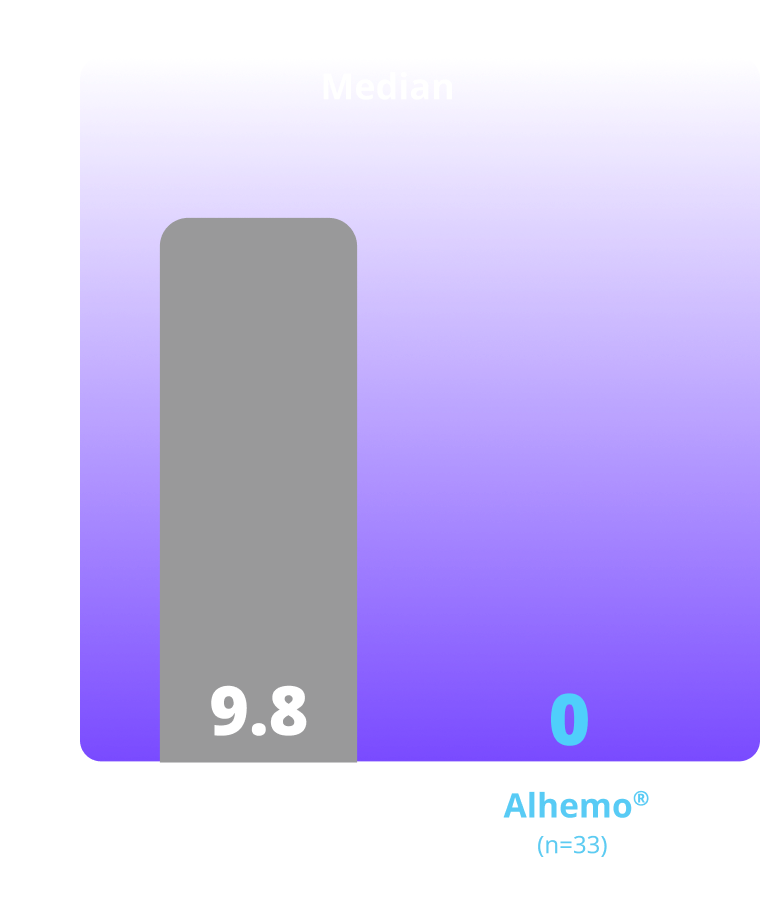
Study design: explorer7 was a multinational, multicenter, open-label, phase 3 trial that investigated the safety and efficacy of Alhemo® for routine PPx in 91 adult males (58 HAwI and 33 HBwI) and 42 adolescent males (22 HAwI and 20 HBwI) who had been prescribed or were in need of treatment with BPAs. Efficacy was evaluated when all patients in arms 1 and 2 had completed at least 24 or at least 32 weeks, respectively, by comparing the number of treated bleeding episodes between Alhemo® PPx (arm 2, n=33) and no PPx treatment (on demand with BPAs; arm 1, n=19). Using a negative binomial model, a ratio of the ABR was estimated to 0.14 (P<0.001).
ABR=annualized bleeding rate; BPA=bypassing agents; HAwI=hemophilia A with inhibitors; HBwI=hemophilia B with inhibitors; IQR=interquartile range; PPx=prophylaxis.
Additional analysis:
Discover lower target joint bleed rate and more bleed-free patients
88% lower bleed rate in target joint
Supportive secondary end point was an estimated mean target joint ABR of 0.1 (95% CI: 0.0-0.9) with Alhemo® (n=33) vs 1.1 (95% CI: 0.3-5.2) with no PPx (n=19), corresponding to estimated mean target joint ABR ratio of 0.123,4,c
Not alpha controlled
64% bleed-free for 24 weeks
for patients on Alhemo® (n=21) vs 10.5% on no prophylaxis (n=2)3,4,d
Additional assessment that is not alpha controlled
aTreated target joint bleeds.
bPatients with zero treated bleeds within first 24 weeks.
ABR=annualized bleeding rate; BPA=bypassing agent; HAwI=hemophilia A with inhibitors; HB=hemophilia B; HBwI=hemophilia B with inhibitors, PPx=prophylaxis.
Individualized dosing
Alhemo® offers individualized dosing for hemophilia patients with and without inhibitors.1
Planning for surgery?
See management recommendations.
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
References:
- Alhemo [package insert]. Plainsboro, NJ; Novo Nordisk Inc.
- Chowdary P, Angchaisuksiri P, Apte S, et al. Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): a prospective, multicentre, open-label, randomised, phase 3a trial. Lancet Haematol. 2024;11(12):e891-e904.
- Matsushita T, Shapiro A, Abraham A, et al. explorer7 Investigators. Phase 3 trial of concizumab in hemophilia with inhibitors. N Engl J Med. 2023;389(9):783-794.
- Matsushita T, Shapiro A, Abraham A, et al. Phase 3 trial of concizumab in hemophilia with inhibitors. Supplementary Appendix. N Engl Med. 2022;389:783-794. Accessed September 3, 2025.
https://www.nejm.org/doi/full/10.1056/NEJMoa2216455

