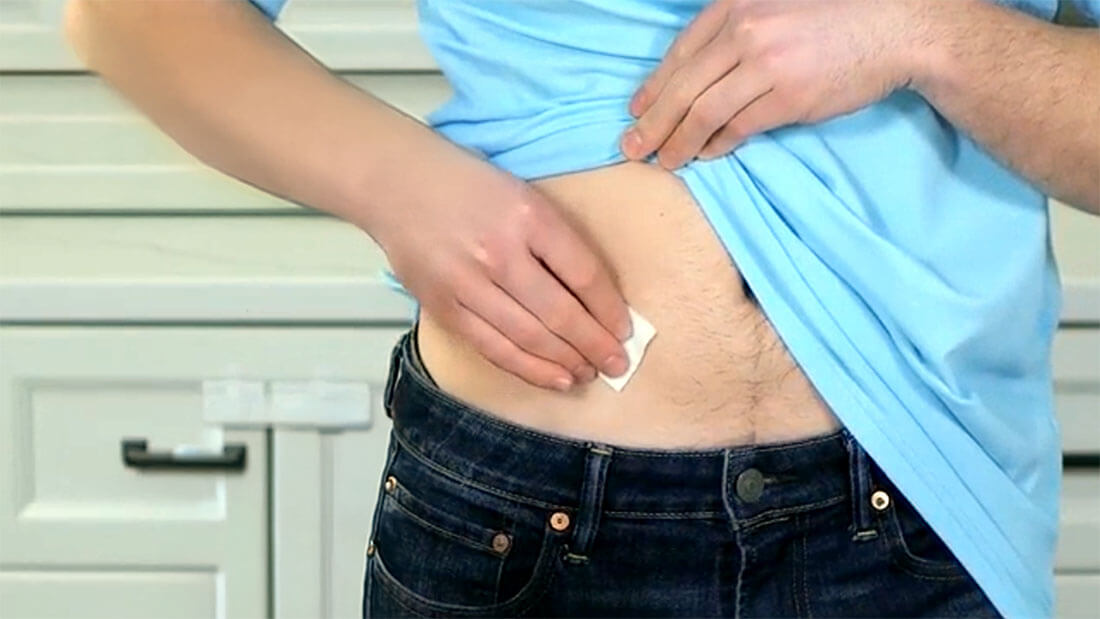
Routine prophylaxis treatment in a prefilled, subcutaneous pen to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older who have hemophilia B and A with or without inhibitors
Administration made easy with the Alhemo® pen1
Treatment is intended for use under the guidance of an HCP and may be self-administered or administered by a caregiver after appropriate training and reading the IFU if a healthcare provider determines that is appropriate.
Actor portrayal.
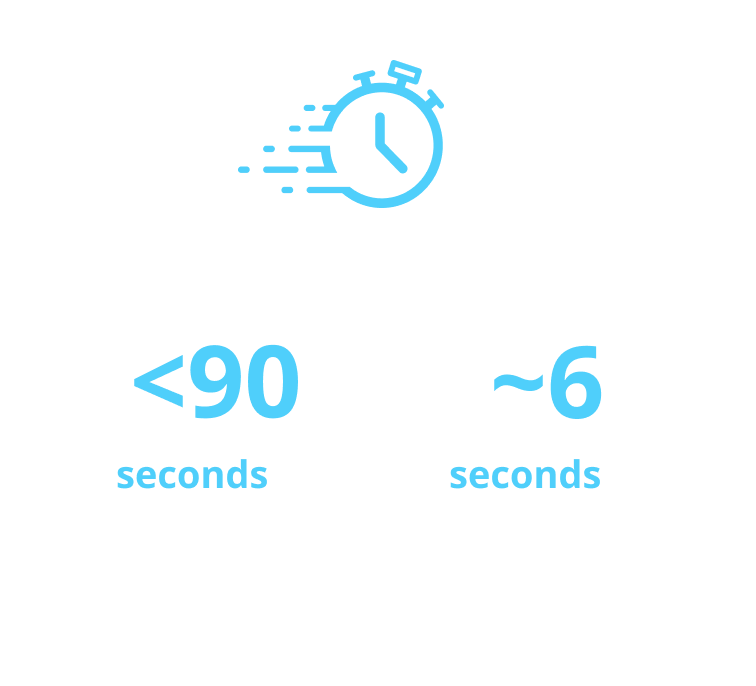

aAfter the dose counter is at “0,” count slowly to 6 while the needle is still in skin.
Study design: In a device-handling study of the Alhemo® pen in 44 adults and 21 adolescents with hemophilia A or B, with or without inhibitors, and 15 caregivers, participants received training and performed a single simulated pen injection into a pad or manikin before completing a questionnaire. Time for training, preparation, injections, and number of complete injections were assessed. 98% (n=78) of participants rated Alhemo® pen easy or very easy to use. Average time to complete an injection was 1 min 21s. May not completely reflect experience of at-home use.
Alhemo® is administered once daily
Patients and caregivers should receive training from an HCP before use and initiate in a nonbleeding state.
Read the Instructions for Use or view the video below.

- Check Alhemo® pen
- Attach new needle

- Prime before dose
Dial 1 marking and test the flow before each dose - Select dose
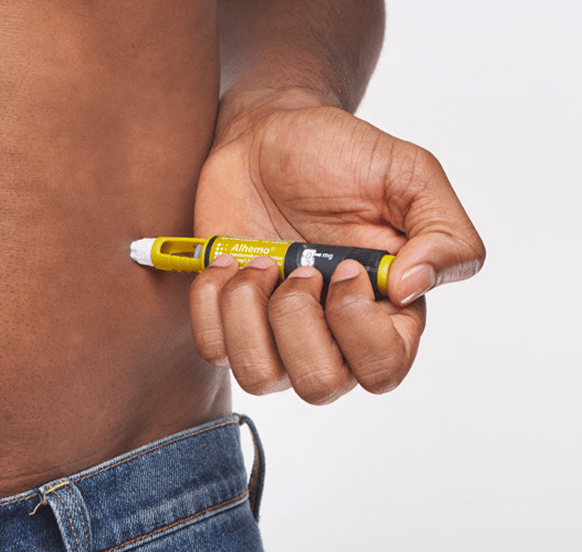
- Prepare injection site
- Inject Alhemo® a,b

- Remove needle
- Recap pen

Needles sold separately and may require a prescription in some states.
aAfter the dose counter is at “0,” count slowly to 6 while the needle is still in skin.
bAdminister by subcutaneous injection to the abdomen or thigh with rotation of injection site every day. Subcutaneous injections should not be given in areas where the skin is tender, bruised, red, or hard, or areas where there are moles, scars, or stretch marks. Each Alhemo® prefilled pen is for use by a single patient.
cStore in refrigerator before first use. After first use, Alhemo® can be stored at room temperature below 86 ℉ (30 ℃) or in a refrigerator at 36 ℉ to 46 ℉ (2 ℃ to 8 ℃) for up to 4 weeks.
dAfter 28 days, discard pen in an FDA-cleared sharps disposal container.
FDA=USA Food and Drug Administration; IFU=Instructions for Use; HCP=healthcare provider.
Alhemo® offers innovative administration1,3
Not intended to be a comparison of efficacy or safety
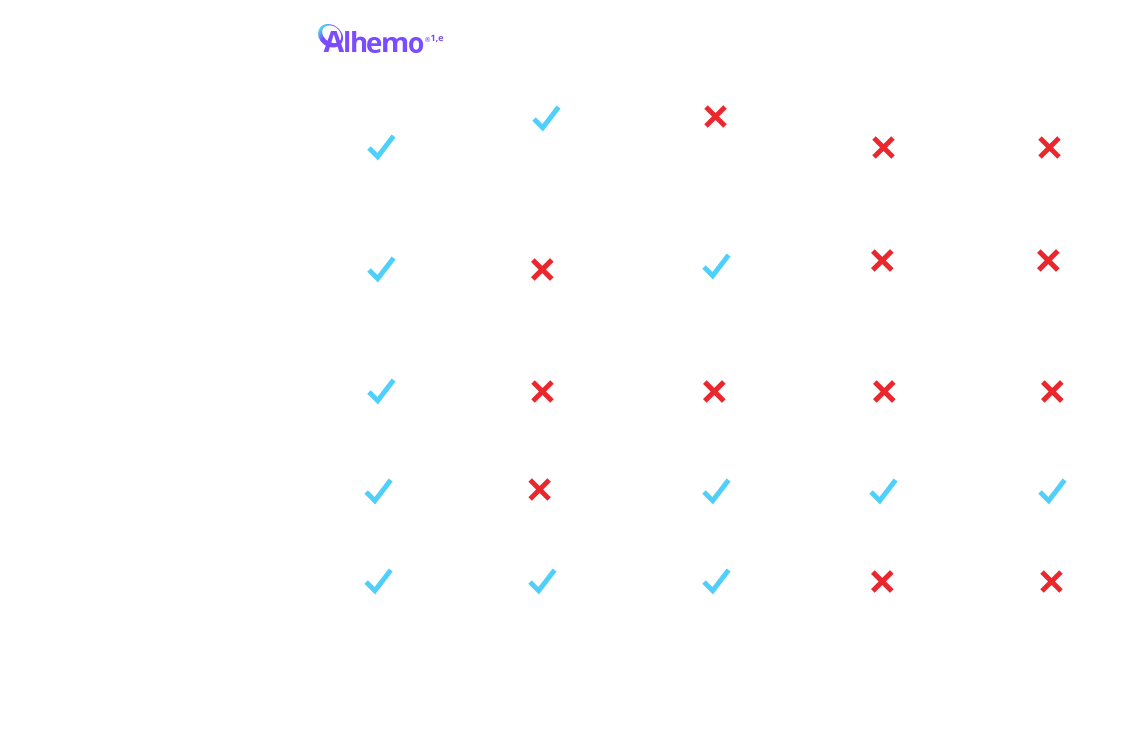
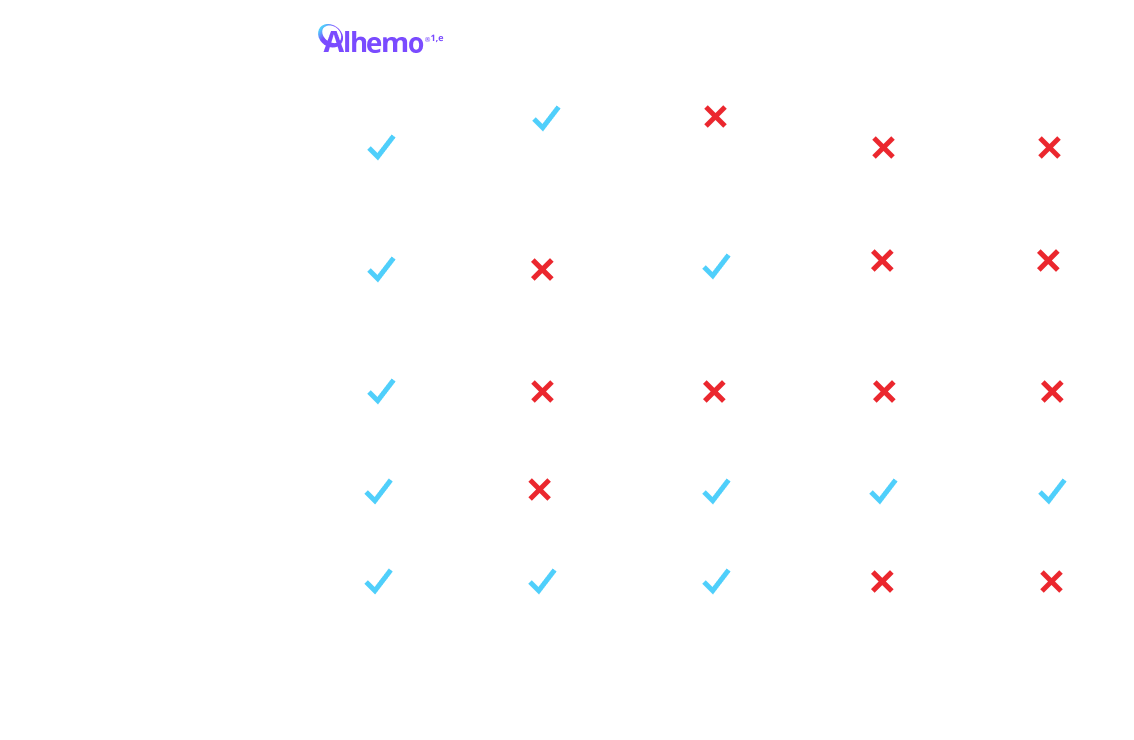
eTreatment is intended under the guidance of an HCP and may be self-administered or administered by a caregiver after appropriate training and reading the IFU, if HCP determines it appropriate.
fFor a patient weighing 70 kg, the volume of HEMLIBRA® to inject ranges from 0.7 mL to 2.8 mL depending on dosing frequency. For the same patient on Alhemo® 0.2 mg/kg, the volume to inject ranges from 0.14 mL to 0.35 mL. depending on pen size. For a patient weighing 70 kg, a single infused dose of NovoSeven® RT for a joint bleed (90 mcg/kg) is 6.3 mL. For a patient weighing 70 kg, a single infused dose of SevenFACT® for a joint bleed (75 mcg/kg) is 5.25 mL. For a patient weighing 70 kg, a single infused dose of FEIBA® for a joint bleed (75 U/kg) is 105 mL. Qfitlia™ volume to inject is 0.5 mL for a 50 mg dose.
EHl=extended half-life; FIX=Factor IX; HB=hemophilia B; HCP=healthcare provider; IFU=Instructions for Use; PPx=prophylaxis; SHl=standard half-life.
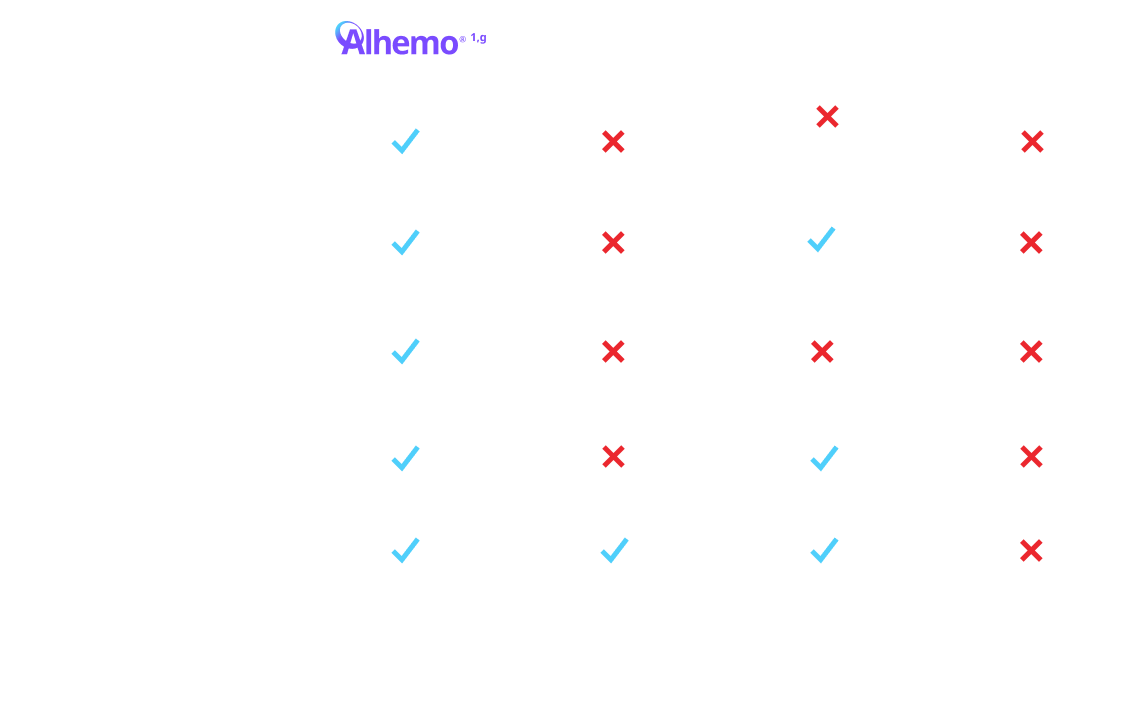
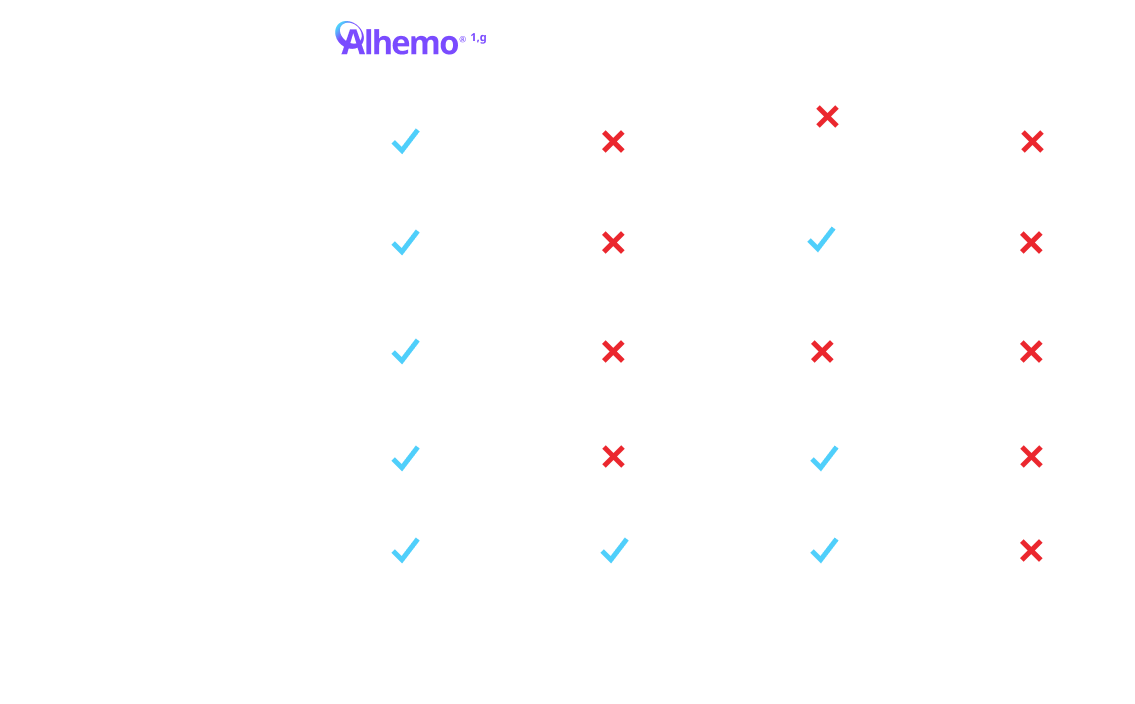
gTreatment is intended under the guidance of an HCP and may be self-administered or administered by a caregiver after appropriate training and reading the IFU, if HCP determines it appropriate.
hFor a patient weighing 70 kg, the volume of Hemlibra® to inject ranges from 0.7 mL to 2.8 mL depending on dosing frequency. For the same patient on Alhemo® 0.2 mg/kg, the volume to inject ranges from 0.14 mL to 0.35 mL, depending on pen size. For a patient weighing 70 kg, a single infused dose of NovoSeven® RT for a joint bleed (90 mcg/kg) is 6.3 mL. For a patient weighing 70 kg, a single infused dose of SevenFACT® for a joint bleed (75 mcg/kg) is 5.25 mL. For a patient weighing 70 kg, a single infused dose of FEIBA® for a joint bleed (75 U/kg) is 105 mL. Qfitlia® volume to inject is 0.5 mL for a 50 mg dose.
EHl=extended half-life; FIX=Factor IX; HB=hemophilia B; HCP=healthcare provider; IFU=Instructions for Use; PPx=prophylaxis; SHl=standard half-life.
Help your patients navigate coverage
NovoCare® is designed for your patients. Patients can access cost savings and support programs.
Stay in the know about Alhemo®
Create a novoMEDLINK™ account and be among the first to receive Alhemo® product updates and information.
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
References:
- Alhemo [package insert]. Plainsboro, NJ; Novo Nordisk Inc.
- Rasmussen NK, Berg B, Christiansen ASL, et al. The concizumab pen-injector is easy to use and preferred by hemophilia patients and caregivers: a usability study assessing pen-injector handling and preference. Patient Prefer Adherence. 2024:18 1713-1727.
- Matsushita T, Shapiro A, Abraham A, et al. Phase 3 trial of concizumab in hemophilia with inhibitors. N Engl J Med. 2023;389(9):783-794
- HYMPAVZI™. Prescribing Information. Pfizer Inc; 2024.
- Qfitlia®[package insert]. Genzyme Corporation; 2025.
- BeneFIX. Prescribing information. Pfizer Inc. Return to TOC 60
- Precision Biologic Inc. Precision Biologic's Chromogenic FIX assay FDA-cleared for sale in U.S. Precision Biologic. Published January 5, 2023. Accessed July 31, 2025. https://precisionbiologic.com/precision-biologics-chromogenic-fix-assay-fda-cleared- for-sale-in-u-s
- Idelvion®. Prescribing Information. CSL Behring LLC; 2023
- World Federation of Hemophilia. Protocols for the treatment of hemophilia and von Willebrand disease. WFH. April 2008. Accessed September 3, 2025. https://www1.wfh.org/publication/files/pdf-1137.pd
- HEMLIBRA® [package insert]. Genentech, Inc; 2024.
- FEIBA® [package insert]. Takeda Pharmaceuticals USA, Inc; 2024.
- NovoSeven® RT [package insert]. Novo Nordisk Inc.
- Sevenfact® [package insert]. Prescribing Information. HEMA Biologics; 2022.