Routine prophylaxis treatment in a prefilled, subcutaneous pen to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older who have hemophilia B and A with or without inhibitors
Guidance to support breakthrough bleed management1
No dose adjustment is required
for breakthrough bleeds
No dose adjustment is required for breakthrough bleeds
Can be used with all BPAs for breakthrough bleedsa
Use the lowest approved dose for mild / moderate bleeds needing rFVIIa or aPCC.
Maximum aPCC dose: 100 units/kg within 24 hours.
For severe bleeds, follow the dosing instructions provided in the approved labeling for the specific product, based on clinical judgment.
aDose and duration will depend on the location and severity of the bleed.
In explorer7, most breakthrough bleeds managed with rFVIIa were treated with 1 injection2
Information provided includes descriptive results from an exploratory analysis at the 56-week cutoff for the explorer7 trial. The 56-week cutoff was defined as when all patients in arms 2, 3, and 4 completed the 56-week visit during the ongoing extension part of the trial (or permanently discontinued treatment).
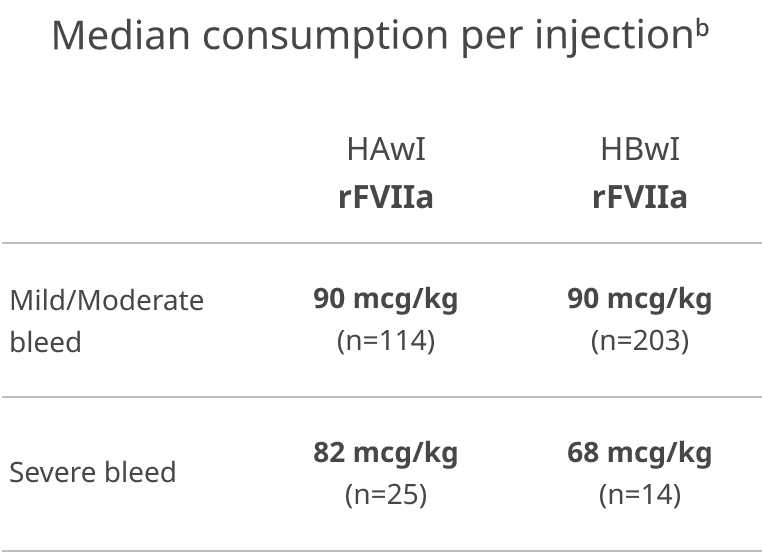
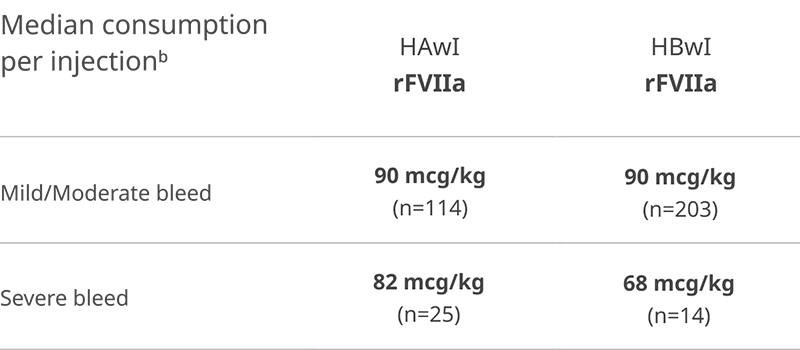
n values = bleeding episodes
bMedian consumption per injection for other treatments. FVIII: HAwI: mild/moderate bleeds (n=11) 20 IU/kg; severe bleeds (n=2) 60 IU/kg. FIX: HBwI: severe bleeds (n=1) 134.4 IU/kg. FVIIa+FX: HBwI: severe bleeds (n=1) 40 mcg/kg. aPCC: HAwI: mild/moderate bleeds (n=43)
50 IU/kg; severe bleeds (n=2) 80 IU/kg. HBwI: mild/moderate bleeds (n=1) 50 IU/kg. In some cases, a bleeding episode was treated with more than one product.
Guidance for treating breakthrough bleeds: Mild to moderate bleeds were treated according to protocol guidance; severe bleeds were treated according to physician’s discretion.2.
Number of mild/moderate bleeding episodes treated with one rFVIIa injection: HAwI (n=114 bleeds): 70 (61.4%); HBwI (n=203 bleeds): 93 (45.8%).
Number of severe bleeding episodes treated with one rFVIIa injection: HAwI (n=25 bleeds): 16 (64%); HBwI (n=14 bleeds): 7 (50%).
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors.3
Important Safety Information
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
See additional Important Safety Information below. See dosing and administration recommendations in the full Prescribing Information, including Boxed Warning.
aPCC=activated prothrombin complex concentrate; FIX=Factor IX; FVIIa=Activated Factor VII; FVIII=Factor VIII; FX=Factor X; HAwI=hemophilia A with inhibitors; HBwI=hemophilia B with inhibitors; rFVIIa=recombinant activated factor VIIa.
Forward-planning management of surgeries1
No dose adjustment is required
for minor surgeries
No dose adjustment is required for minor surgeries
Management of Alhemo® in major surgeriesa
As there is limited experience in the perioperative setting:

4 days
prior to major surgery

10-14 days
after surgery with same maintenance dose (no loading dose) while considering the overall clinical picture of the patient
Reaches steady state and washes out quickly, within 4 days.d
dFollowing a single Alhemo® loading dose of 1 mg/kg, the steady state concentrations were reached around Day 4 and remained within a stable exposure range with daily maintenance doses. Based on population pharmacokinetic analysis, 90% of concizumab-mtci is expected to be eliminated by the end of approximately 4 days after the last dose (time for 50% of drug to be eliminated is approximately 1 day).
Surgeries performed during explorer7 clinical trial2
Minor surgeries included:
- Dental procedure (n=7, HAwI) (n=2, HBwI)
- Port removal (n=1, HBwl)
- Embolization of the right femoral artery for an SAE (recorded as retroperitoneal hematoma with active lumbar arterial spread after traumatic injury) (n=1, HAwl)
- Tongue mucous membrane injury (n=1, HBwl)
- Circumcision (phimosis) (n=1, HAwl)
- Venesection to perform infusion therapy in right foot (n=1, HAwl)
Major surgeries included:
- Hip arthroplasty (left hip hemophilic arthropathy) (n=1, HAwI)
- Total hip arthroplasty (right femoral neck fracture) (n=1, HBwI)
Information provided includes descriptive results from the 56-week cutoff for the explorer7 trial. The 56- week cutoff was defined as when all patients in arms 2, 3, and 4 completed the 56-week visit during the ongoing extension part of the trial (or permanently discontinued treatment).
Alhemo® is not indicated for perioperative management.1
HAwI=hemophilia A with inhibitors; HBwI=hemophilia B with inhibitors; SAE=serious adverse event.
The first TFPI antagonist for HBwI and HAwI1
Discover Alhemo®’s Mechanism of Action (MoA).
Individualized dosing for HBwI and HAwI
For your hemophilia patients with inhibitors, Alhemo® offers individualized dosing.1
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Important Safety Information for NovoSeven® RT
WARNING: THROMBOSIS
- Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported
- Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT
- Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis
Warnings and Precautions
- Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance
- Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events
- Hypersensitivity reactions, including anaphylaxis, can occur with NovoSeven® RT. Patients with a known hypersensitivity to mouse, hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions. Discontinue infusion and administer appropriate treatment when hypersensitivity reactions occur
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level, or PT is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis
Adverse Reactions
- The most common and serious adverse reactions in clinical trials are thrombotic events. Thrombotic adverse reactions following the administration of NovoSeven® RT in clinical trials occurred in 4% of patients with acquired hemophilia and 0.2% of bleeding episodes in patients with congenital hemophilia
Drug Interactions
- Thrombosis may occur if NovoSeven® RT is administered concomitantly with Coagulation Factor XIII
Please click here for NovoSeven® RT Prescribing Information, including Boxed Warning.
Indications and Usage
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor indicated for:
- Treatment of bleeding episodes and perioperative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets
- Treatment of bleeding episodes and perioperative management in adults with acquired hemophilia
Important Safety Information for Alhemo®
Contraindications
- Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients
Warnings and Precautions
- Thromboembolic Events (TEs): Venous and arterial TEs were reported in 1.9% of patients (6/320) who also had multiple risk factors, including the use of high doses or prolonged treatment with factor product or bypassing agent (2 of 6 patients). Risk factors for TEs may also include conditions in which tissue factor is overexpressed (eg, atherosclerotic disease, crush injury, cancer, disseminated intravascular coagulation, thrombotic microangiopathy, or septicemia). Inform patients about and monitor them for signs and symptoms of TEs. In case of suspicion of TEs, discontinue Alhemo® and initiate further investigations and management strategies
- Hypersensitivity Reactions: Alhemo® is contraindicated in patients with a history of known serious hypersensitivity to Alhemo® or its ingredients. Hypersensitivity reactions, including erythema, rash, pruritus, and abdominal pain, have occurred in patients treated with Alhemo®. One patient (<1%) experienced anaphylaxis, which resolved after treatment with antihistamines and corticosteroids. Instruct patients of the signs of acute hypersensitivity reactions and to contact their healthcare provider for mild reactions and to seek urgent medical attention for moderate to severe reactions. Discontinue Alhemo® if severe hypersensitivity symptoms occur and initiate medical management
- Increased Laboratory Values of Fibrin D-dimer and Prothrombin Fragment 1.2: Increased levels of fibrin D-dimer and prothrombin fragment 1.2 were seen in 29 (9.1%) and 26 (8.1%) patients, respectively, which is positively correlated with the plasma concentration of concizumab-mtci, indicating a hemostatic effect. For patients taking Alhemo®, these coagulation biomarkers may not be reliable predictive markers for clinical decision-making with suspicion of thrombosis, such as deep vein thrombosis and pulmonary embolism
Adverse Reactions
- The most frequently reported adverse reactions (≥5%) were injection site reactions, headache, and urticaria
- Serious adverse reactions were reported in 6.1% of patients with inhibitors who received Alhemo®. Permanent discontinuation of Alhemo® occurred in 1 patient due to a renal infarct and dosage interruptions of Alhemo® occurred in 1 patient (3%) and was a hypersensitivity reaction
Drug Interactions
- Breakthrough Bleeding Treatment: Take appropriate precautions when treating breakthrough bleeding events in patients receiving Alhemo® prophylaxis and FVIII or FIX or a bypassing agent (eg, rFVIIa or aPCC). For mild and moderate bleeds, the lowest approved dose in the approved product labeling is recommended. For aPCC, a maximum dose of 100 units/kg within 24 hours is recommended. For severe bleeds, follow the dosing instructions in the approved labeling based on clinical judgment
Please click here for Alhemo® Prescribing Information.
Indications and Usage
Alhemo® (concizumab-mtci) injection 60 mg, 150 mg, or 300 mg is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
Reference:
- Alhemo [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Data on file. Novo Nordisk Inc; Plainsboro, NJ.
- NovoSeven® RT [package insert]. Novo Nordisk Inc.


