For pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone (GH) and Prader-Willi Syndrome; short stature associated with Noonan Syndrome, Turner Syndrome, and children born small for gestational age; idiopathic short stature; and for the replacement of endogenous GH in adults with growth hormone deficiency (GHD). Please see full indications.
Increased height in pediatric patients with growth hormone deficiency (GHD).
Increased lean body mass and lowered total body fat compared to placebo in adults with GHD1
Increased height in pediatric patients with growth hormone deficiency (GHD).
Increased lean body mass and lowered total body fat compared to placebo in adults with GHD1
Actor portrayal

Significantly greater change in standing height from baseline to month 12 and month 24 with 0.05 or 0.1 mg/kg/day vs 0.025 mg/kg/day in pediatric patients with GHD1
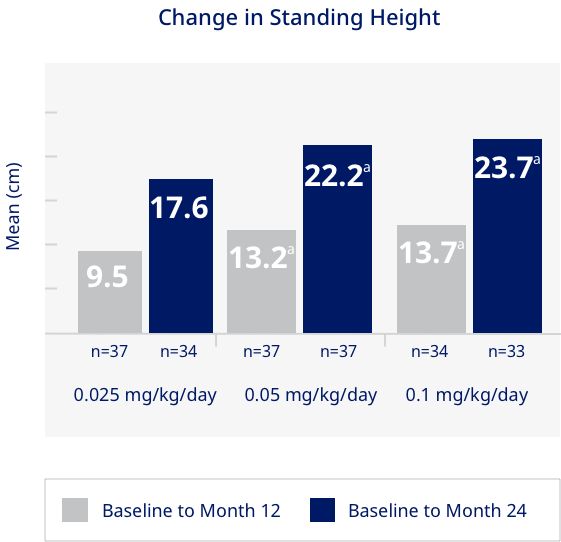
- Adjusted mean increases in HSDS over the 2-year period were 0.81, 1.57, and 1.73 in the 0.025, 0.05, and 0.1 mg/kg/day dose groups, respectively1
- Height velocity (cm/year) and HVSDS increased considerably after initiation of treatment, with the greatest response observed during the first year of treatment1
- Mean change in sitting height (cm) from baseline to month 24 was significant (P<0.05) compared to both other groups for 0.05 mg/kg/day and 0.1 mg/kg/day: 9.3 (0.025 mg/kg/day; n=29), 10.8 (0.05 mg/kg/day; n=35), and 12.2 (0.1 mg/kg/day; n=31)1
HV = height velocity; HSDS = height standard deviation score.
aSignificant (P<0.05) change from baseline compared to the 0.025 mg/kg/day group.
Norditropin® Clinical Study: Growth Failure due to Inadequate Secretion of Endogenous Growth Hormone
The efficacy and safety of Norditropin® was assessed in a multicenter, prospective randomized, open label, dose response study with three doses (0.025, 0.05, and 0.1 mg/kg/day). A total of 111 pediatric patients with GHD were randomized to each dose; 37(0.025 mg/kg/day):38(0.05 mg/kg/day):36(0.1 mg/kg/day). Patients met the following entry criteria: chronological age ≥ 3 years with a skeletal age < 10 years if male and < 8 years if female; pubertal stage = stage 1; previously untreated GHD; peak plasma hormone concentration < 7 ng/mL or < 10 ng/mL (depending on assay used) in two tests.1
Significantly higher lean body mass and lower total body fat in adult GHD patients treated with Norditropin® vs Placebo1
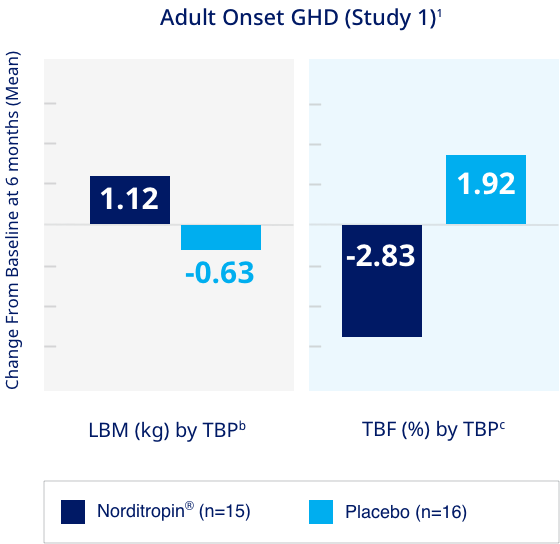
Study Design (Study 1):
A single center, randomized, double-blind, placebo-controlled, parallel-group, six-month clinical trial was conducted in 31 adults with adult onset GHD comparing the effects of Norditropin® (somatropin) injection and placebo on body composition. Patients in the active treatment arm were treated with Norditropin® 0.017 mg/kg/day (not to exceed 1.33 mg/day). The changes from baseline in LBM and TBF were measured by TBP after 6 months.1
LBM = lean body mass; TBP = total body potassium; TBF = total body fat; CI = confidence interval; ANOVA = analysis of variance.
bNorditropin® vs. placebo (95% CI): +1.74 (0.65, 2.83) P=0.0028. Least square mean based on an ANOVA model including treatment and sex as factors.1
cNorditropin® vs. placebo (95% CI): -4.74 (-7.18, -2.30) P=0.0004. Least square mean based on an ANOVA model including treatment and sex as factors.1
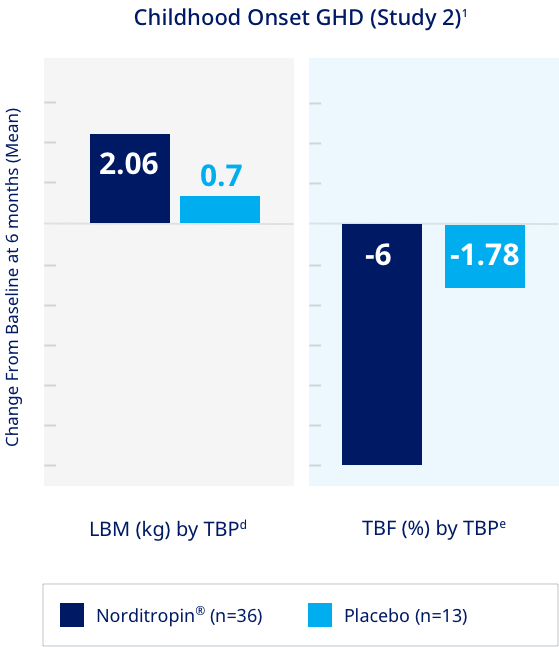
Study Design (Study 2):
A single center, randomized, double-blind, placebo-controlled, parallel-group, dose-finding, six-month clinical trial was conducted in 49 men with childhood onset GHD comparing the effects of Norditropin® and placebo on body composition. Patients were randomized to placebo or one of three active treatment groups (0.008, 0.016, and 0.024 mg/kg/day). Thirty-three percent of the total dose to which each patient was randomized was administered during weeks 1-4, 67% during weeks 5-8, and 100% for the remainder of the study. The changes from baseline in LBM and percent TBF were measured by TBP after 6 months.1
dNorditropin® vs placebo (95% CI): +1.4 (0.39, 2.41) P=0.0079. Least square mean based on an ANOVA model including treatment as a factor.1
eNorditropin® vs placebo (95% CI): -4.24 (-7.11, -1.37) P=0.0048. Least square mean based on an ANOVA model including treatment as a factor.1
Looking for data about other growth-related disorders?
Explore clinical studies which included pediatric patients diagnosed with idiopathic short stature, Noonan syndrome, Turner syndrome, born small for gestational age, and Prader-Willi syndrome.
How did Norditropin® perform in a real-world-setting?
View safety and efficacy results from an observational study of pediatric patients in the U.S. (ANSWER–originally a post-marketing registry).2,a
aThe ANSWER Program was originally a post-marketing registry of adults and pediatric patients treated with Norditropin® as prescribed by their physician and according to routine clinical practice. Since it did not investigate treatment specifics, it was developed into a non-interventional, observational study that allowed evaluation of the safety and effectiveness of Norditropin® in a real-world setting in the United States.2
Individualized treatment
Norditropin® FlexPro® pens allow you to personalize your patients’ dosing regimens from childhood through adolescence.1
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, 15 mg, or 30 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
References:
- Norditropin [prescribing information]. Plainsboro, NJ: Novo Nordisk Inc.
- Data on file. Novo Nordisk Inc; Plainsboro, NJ