For pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone (GH) and Prader-Willi Syndrome; short stature associated with Noonan Syndrome, Turner Syndrome, and children born small for gestational age; idiopathic short stature; and for the replacement of endogenous GH in adults with growth hormone deficiency (GHD). Please see full indications.
Supporting growth-related disorders for 25 years and counting1
Norditropin® was first approved by the FDA in 1995.1
Supporting growth for 25 years and counting1
Norditropin® was first approved by the FDA in 1995.1
Actor portrayal

How Norditropin® indications compare to other once-daily therapies1-7,a
More indications to help more of your patients.
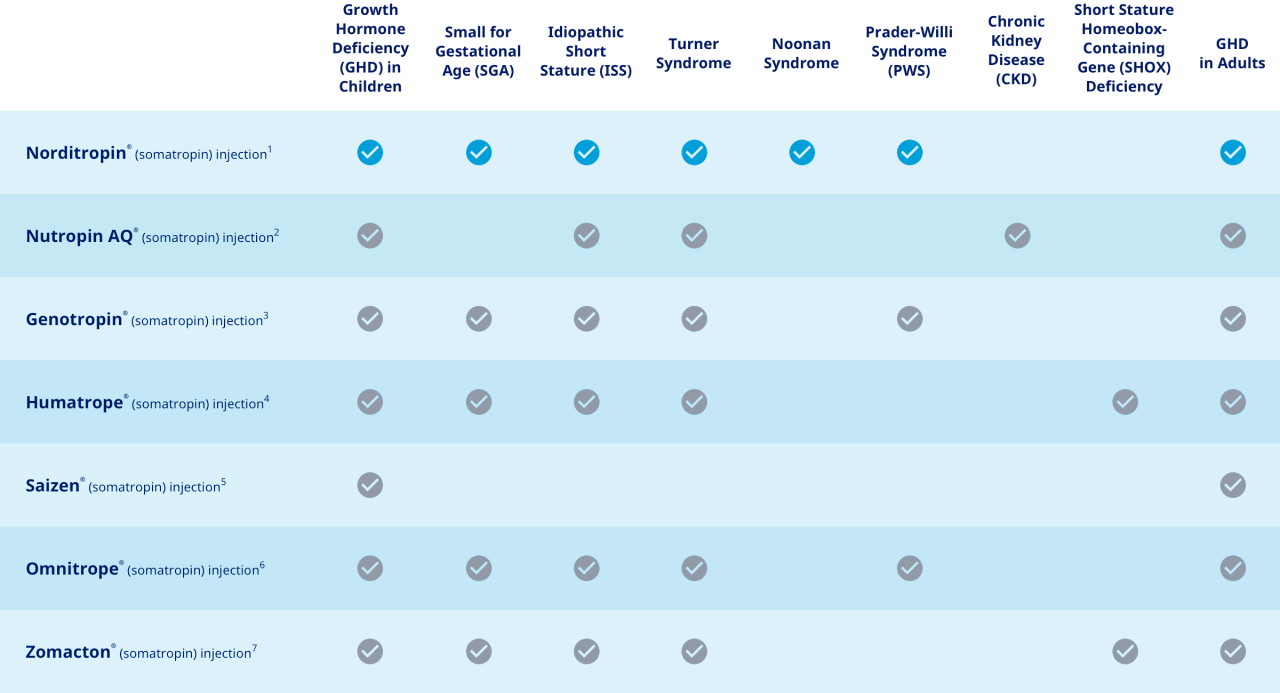

aThis chart is not intended to be a comparison of efficacy or safety.
How Norditropin® (somatropin) injection works to improve height
Somatropin binds to dimeric growth-hormone receptors located within the cell membranes of target tissue cells. This interaction results in intracellular signal transduction and subsequent induction of transcription and translation of GH-dependent proteins including IGF-1. Somatropin has direct tissue and metabolic effects or mediated indirectly by IGF-1, including stimulation of chondrocyte differentiation and proliferation, and protein synthesis.1
Somatropin stimulates skeletal growth in pediatric patients with GHD as a result of effects on the growth plates (epiphyses) of long bones, leading to increased linear growth rate (height velocity) in most cases. Linear growth is facilitated in part by increased cellular protein synthesis.1
See Prescribing Information for complete description.

What’s the right dose for your patients?
Norditropin® FlexPro® prefilled pens are available in strengths of 5 mg, 10 mg, 15 mg, and 30 mg. The daily dose for pediatric patients is based on the patient’s indication and weight.1
Easy to learn to use and designed with patients in mind8,b,c

- Prefilled, premixed, and preloaded1
- Easy-to-push dose button8,c
- Multidose1
- 19mm diameter8
- 32-gauge needled
- Optional PenMate® accessory keeps needle out of sight1,e
In a usability study, participants reported feeling comfortable using the FlexPro® pen.8,f
missed injections in a typical month as reported in a web-based survey
by 76% of patients/caregivers using a storage-flexible GH9,g
missed injections in a typical month as reported in a web-based survey
by 76% of patients/caregivers using a storage-flexible GH9,g
bPlease see Instructions for Use for complete instructions.
cBased on a human factors study of the safety and usability of the Norditropin® FlexPro® 30 mg pen in 94 participants (children ages 10-17 with growth-related disorders; adults with GHD; HCPs; and caregivers). Participants, excluding inpatient nurses, received training and performed injections using a foam cushion and then completed a device-specific questionnaire. Participants rated the device a 6.7 out of 7 (range 5 to 7 on a scale of 1 to 7, where 1 means “strongly disagree” and 7 means “strongly agree”) for the statement, "FlexPro® was easy to learn to use.”8
dNeedles are sold separately and may require a prescription in some states.
ePenMate® is a reusable accessory available for Norditropin® FlexPro® 5, 10, and 15 mg pens only.1
fBased on a human factors study of the safety and usability of the Norditropin® FlexPro® 30 mg pen in 94 participants (children ages 10-17 with growth-related disorders; adults with GHD; HCPs; and caregivers). Participants, excluding inpatient nurses, received training and performed injections using a foam cushion and then completed a device-specific questionnaire. When asked if they agreed with the statement “I felt comfortable using FlexPro,” participants responded with a mean rating of 6.7 out of 7 (range 4 to 7 on a scale of 1 to 7, where 1 means “strongly disagree” and 7 means “strongly agree”).8
gThis study surveyed 146 respondents (48 patients, 98 caregivers) with experience using one type of GH product (storage-flexible or refrigeration-only). Respondents provided feedback on their GH product with respect to the basic GH therapy overview, typical injection process, how they manage their injection when special occasions or other activities arise, and wastage and storage. The functional and emotional impact of storage-flexible GH products vs that of refrigeration-only GH products on patients’ and caregivers’ daily lives was assessed using a web-based survey.9
Room temperature stable for up to 21 days after first use1
After first injection, Norditropin® pens can either be stored outside of the refrigerator (up to 77°F) for use within 3 weeks, or in the refrigerator (between 36°F and 46°F) for use within 4 weeks. All unused Norditropin® FlexPro® prefilled pens must be refrigerated (36°F to 46°F) prior to first use. Do not freeze, and avoid direct light.1
Learn about the features of Norditropin® FlexPro® and other devices
Our device tool helps show some of the differences between the FlexPro® device and others on the market.

Broad coverage for commercial and Medicaid patients8
Norditropin® has the broadest coverage in its class.1,i See coverage in your area with one quick search.
iAs of March 2023, based on data from IQVIA and MMIT.8
Questions about Norditropin®?
Study design
(Kappelgaard et al) Web-based Survey Assessing the Impact of Storage Flexibility on the Daily Life of Patients and Caregivers Administering GH
Participants:
Survey respondents (N=146; 48 patients, 98 caregivers) who had experience using only one type of GH product (storage-flexible or refrigeration-only) were included. In order to qualify for the study, patients (≥5 to ≤25 years) or their caregivers were required to use a pen device to administer GH for a growth-related disorder (more than once per week but no more than once daily) and be involved in either the injection itself or in managing the materials required for injection. Caregivers completed the survey for patients <14 years. In the United States, patients 14 to 17 years of age were required to have their caregiver present during the survey. In Europe, caregivers were required to be present during the interview or give consent.9 The classification of GH product type for each patient was determined based on screening question responses. Storage-flexible products included Norditropin® FlexPro®, Norditropin® NordiPen®, and Genotropin® MiniQuick® while refrigeration-only products included Omnitrope® Pen, Humatrope®, Genotropin®, Saizen®, Nutropin AQ®, Nutropin AQ® NuSpin™, easypod®, Tev-Tropin®/Tjet®, Genotropin GoQuick®, one.click®, and other pens (from Pfizer or Lilly).9
Methods:
The survey was conducted from April 2014 to June 2014 in France, Germany, Italy, and the United States. Respondents completed a 20-minute, computer-assisted, web-based interview at home. Interviewers were aware of the sponsor, but patients and caregivers were blinded to reduce potential bias. They were allowed to discuss the questions with the interviewer by telephone as they proceeded through the survey or could complete the survey by web only without assistance. The questionnaire comprised 4 sections, with a maximum of 66 total questions: basic GH therapy overview, typical injection process, special occasions (weddings, vacations) or activities (overnight stays, social occasions, school/work trips), and wastage and storage.9
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administered subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, or 15 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, or 15 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administered subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, or 15 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administered subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administered subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, or 15 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Indications and Usage
Norditropin® (somatropin) injection 5 mg, 10 mg, or 15 mg is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome
- short stature associated with Turner syndrome
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropins
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropins.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients with short stature receiving somatropin, including Norditropin®. Evaluate pediatric patients receiving Norditropin® with the onset of a limp or complaints of hip or knee pain for slipped capital femoral epiphysis and osteonecrosis and manage accordingly.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administered subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies
Please click here for Norditropin® Prescribing Information.
- Norditropin [prescribing information]. Plainsboro, NJ: Novo Nordisk Inc.
- Nutropin AQ [prescribing information]. San Francisco, CA: Genentech, Inc; 2016.
- Genotropin [prescribing information]. New York, NY: Pharmacia & Upjohn Co; 2019.
- Humatrope [prescribing information]. Indianapolis, IN: Eli Lilly & Co; 2019.
- Saizen [prescribing information]. Rockland, MA: EMD Serono, Inc; 2020.
- Omnitrope [prescribing information]. Princeton, NJ: Sandoz, Inc; 2019.
- Zomacton [prescribing information]. Parsippany, NJ: Ferring Pharmaceuticals, Inc; 2018.
- Data on File. Novo Nordisk, Inc.; Plainsboro, NJ.
- Kappelgaard AM, Metzinger CP, Schnabel D. A web-based survey assessing the impact of storage flexibility on the daily life of patients and caregivers administering growth hormone. Expert Rev Med Devices. 2015;12(5):517-527.