NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart) injectable suspension 100 U/mL. NovoLog® Mix 70/30 is a mixture of insulin aspart protamine and insulin aspart indicated to improve glycemic control in adults with diabetes.
Simple 1:1 unit conversion. More flexible dosing.
Convert patients from human premixed insulin (eg, Novolin® 70/30) to NovoLog® Mix 70/30 using a simple 1:1 unit conversion

The INITIATE Study: starting dose and titration
The INITIATE study shows an option for starting dose and titration for patients with type 2 diabetes who are starting insulin therapy with NovoLog® Mix 70/30.
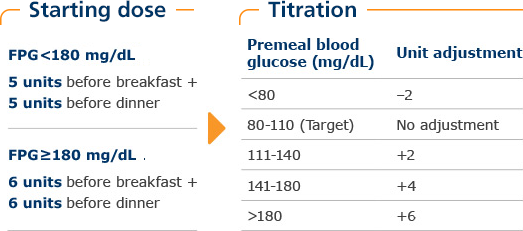
Results from a 28-week, randomized, multicenter, open-label, parallel-group, treat-to-target study with a 4-week metformin optimization period with or without thiazolidinediones. There were 233 insulin-naïve patients with type 2 diabetes who were randomized to either twice-daily NovoLog® Mix 70/30 before breakfast and dinner or to once-daily insulin glargine at bedtime. Primary endpoint was reduction in A1C values from baseline to end of study.1
66% of patients reached A1C goal <7% with twice-daily dosing of NovoLog® Mix 70/30 compared with 40% with once-daily glargine (P<0.001)1
In the INITIATE study, weekly dose adjustments were made for the first 12 weeks and biweekly adjustments thereafter to achieve target FPG (fasting plasma glucose) and predinner blood glucose of 80 to 110 mg/dL. Adjustments were based on self-measured plasma glucose (SMPG) readings (prebreakfast or predinner) from the previous 3 days. If 2 of the 3 readings were not within target, dose adjustment was based on the lowest reading. Predinner doses were titrated based on FPG values. Prebreakfast doses were titrated based on predinner SMPG values.1
Dosing of NovoLog® Mix 70/30 must be individualized.
Every minute counts
NovoLog® Mix 70/30 can be dosed within 15 minutes before or after starting a meal in adult patients with type 2 diabetes, compared with 30 minutes before a meal required by human premixed insulin.2-4
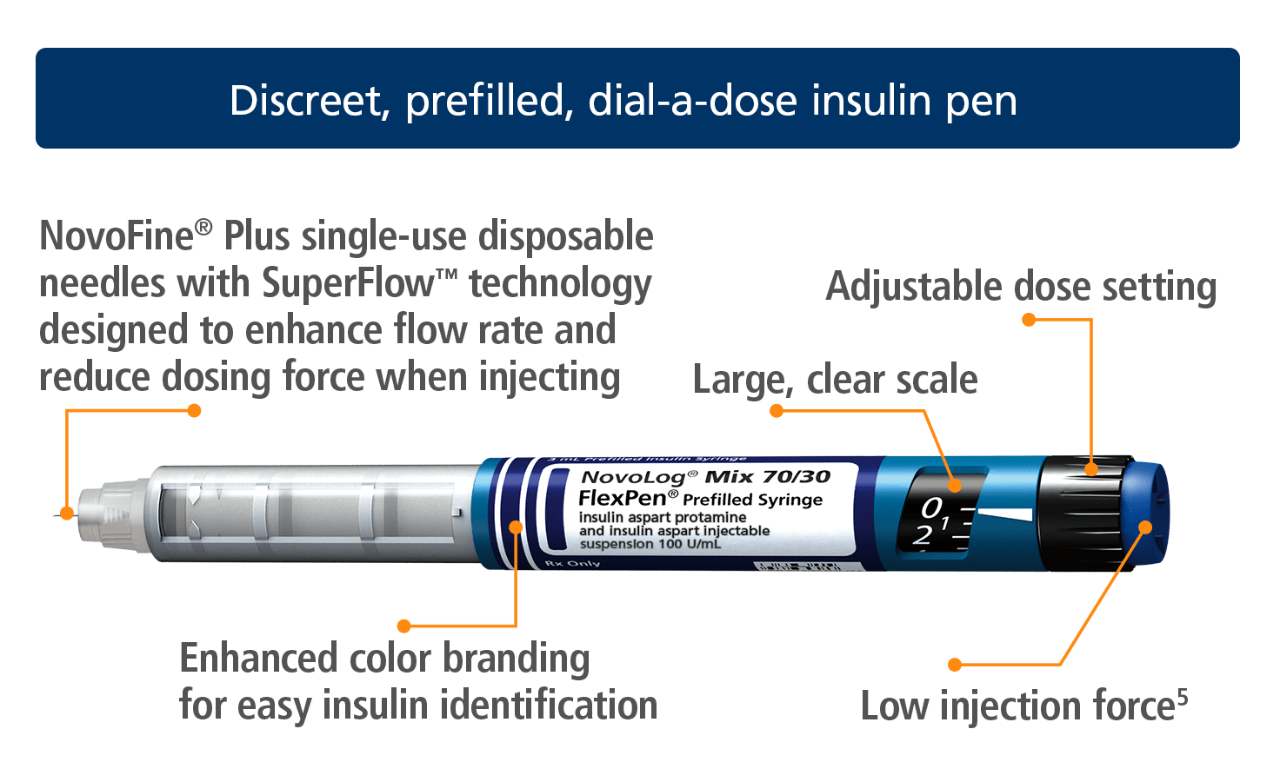
The features of NovoLog® Mix 70/30 FlexPen®
Needles are sold separately and may require a prescription in some states.

Additional features of NovoLog® Mix 70/30 FlexPen®
- Teachable, learnable, usable5
- Proven accuracy6
- Discreet—fits neatly in your patient’s purse or pocket
Important Safety Information for NovoLog® Mix 70/30
Contraindications
- NovoLog® Mix 70/30 is contraindicated during episodes of hypoglycemia and in patients hypersensitive to NovoLog® Mix 70/30 or one of its excipients.
Warnings and Precautions
- Never Share a NovoLog® Mix 70/30 FlexPen®, Needle, or Syringe Between Patients, even if the needle is changed. Patients using NovoLog® Mix 70/30 vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse effect of all insulins, including NovoLog® Mix 70/30. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers), or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia: The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of NovoLog® Mix 70/30 may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to co-administered medication. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. - Hypoglycemia Due to Medication Errors: To avoid medication errors and accidental mix-ups between NovoLog® Mix 70/30 and other insulins, instruct patients to always check the insulin label before injection.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulins, including NovoLog® Mix 70/30. If hypersensitivity reactions occur, discontinue NovoLog® Mix 70/30; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including NovoLog® Mix 70/30, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentration).
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including NovoLog® Mix 70/30. Patients should be observed for signs and symptoms of heart failure. If heart failure develops, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions observed with insulin therapy include hypoglycemia, allergic reactions, local injection site reactions, lipodystrophy, rash, and pruritus.
Use in Specific Populations
- The safety and effectiveness of NovoLog® Mix 70/30 have not been established in pediatric patients. Clinical studies of NovoLog® Mix 70/30 did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
Drug Interactions
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine.
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine.
Please click here for NovoLog® Mix 70/30 Prescribing Information.
Indications and Usage
- NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart) injectable suspension 100 U/mL is a mixture of insulin aspart protamine and insulin aspart indicated to improve glycemic control in adult patients with diabetes mellitus.
Important Limitations of Use
- NovoLog® Mix 70/30 is not recommended for the treatment of diabetic ketoacidosis.
- The proportions of rapid-acting and long-acting insulins are fixed and do not allow for basal versus prandial dose adjustments.
Important Safety Information for NovoLog® Mix 70/30
Contraindications
- NovoLog® Mix 70/30 is contraindicated during episodes of hypoglycemia and in patients hypersensitive to NovoLog® Mix 70/30 or one of its excipients.
Warnings and Precautions
- Never Share a NovoLog® Mix 70/30 FlexPen®, Needle, or Syringe Between Patients, even if the needle is changed. Patients using NovoLog® Mix 70/30 vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, or injection site or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. Adjustments in concomitant anti-diabetic treatment may be needed.
- Hypoglycemia: Hypoglycemia is the most common adverse effect of all insulins, including NovoLog® Mix 70/30. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers), or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia: The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of NovoLog® Mix 70/30 may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature. Other factors which may increase the risk of hypoglycemia include changes in meal pattern, changes in level of physical activity, or changes to co-administered medication. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia. Patients and caregivers must be educated to recognize and manage hypoglycemia. - Hypoglycemia Due to Medication Errors: To avoid medication errors and accidental mix-ups between NovoLog® Mix 70/30 and other insulins, instruct patients to always check the insulin label before injection.
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with insulins, including NovoLog® Mix 70/30. If hypersensitivity reactions occur, discontinue NovoLog® Mix 70/30; treat per standard of care and monitor until symptoms and signs resolve.
- Hypokalemia: All insulins, including NovoLog® Mix 70/30, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentration).
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs), which are PPAR-gamma agonists, and insulin, including NovoLog® Mix 70/30. Patients should be observed for signs and symptoms of heart failure. If heart failure develops, dosage reduction or discontinuation of the TZD must be considered.
Adverse Reactions
- Adverse reactions observed with insulin therapy include hypoglycemia, allergic reactions, local injection site reactions, lipodystrophy, rash, and pruritus.
Use in Specific Populations
- The safety and effectiveness of NovoLog® Mix 70/30 have not been established in pediatric patients. Clinical studies of NovoLog® Mix 70/30 did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
Drug Interactions
- Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics.
- Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
- Drugs that may increase or decrease the blood glucose lowering effect: alcohol, beta-blockers, clonidine, lithium salts, and pentamidine.
- Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine.
Please click here for NovoLog® Mix 70/30 Prescribing Information.
Indications and Usage
- NovoLog® Mix 70/30 (insulin aspart protamine and insulin aspart) injectable suspension 100 U/mL is a mixture of insulin aspart protamine and insulin aspart indicated to improve glycemic control in adult patients with diabetes mellitus.
Important Limitations of Use
- NovoLog® Mix 70/30 is not recommended for the treatment of diabetic ketoacidosis.
- The proportions of rapid-acting and long-acting insulins are fixed and do not allow for basal versus prandial dose adjustments.
References
- Raskin P, Allen E, Hollander P, et al; for the INITIATE Study Group. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260-265.
- NovoLog® Mix 70/30 [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Novolin® 70/30 [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Humulin 70/30 [package insert]. Indianapolis, IN: Eli Lilly and Company, June 2022.
- Reimer T, Hohberg C, Pfützner AH, et al. Intuitiveness, instruction time, and patient acceptance of a prefilled insulin delivery device and a reusable insulin delivery device in a randomized, open-label, crossover handling study in patients with type 2 diabetes. Clin Ther. 2008 Dec; 30(12):2252-2262.
- Pfützner A, Reimer T, Hohberg C, Frøkjær LPF, Jørgensen C. Prefilled insulin device with reduced injection force: patient perception and accuracy. Curr Med Res Opin. 2008;24(9):2545-2549.