Rivfloza® is uniquely designed to reduce UOx excretion2
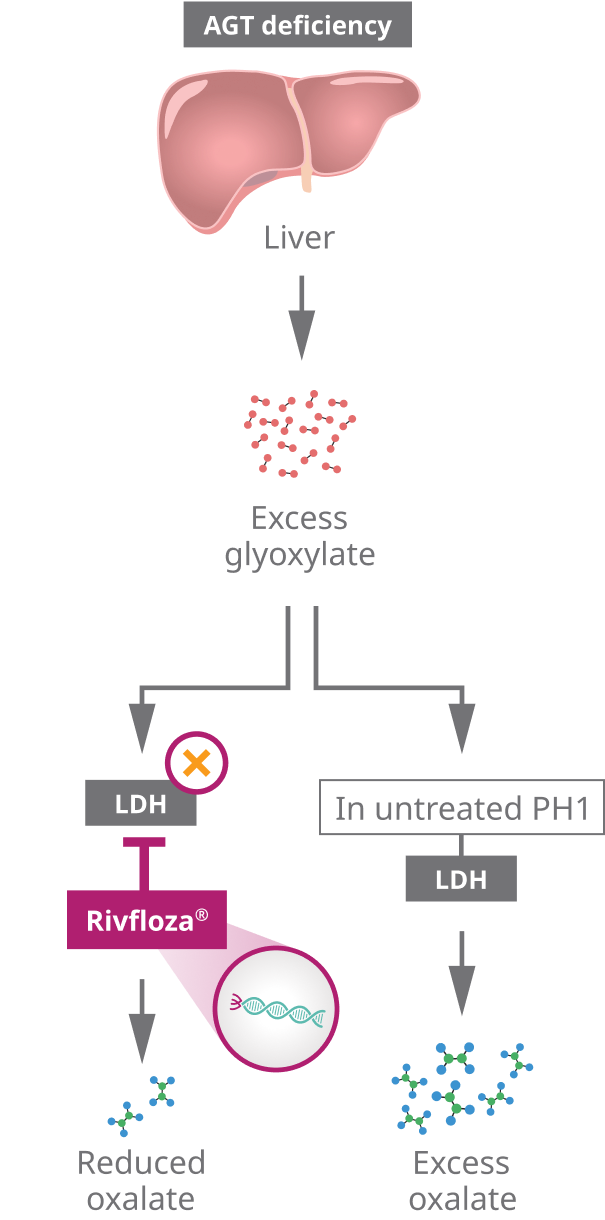
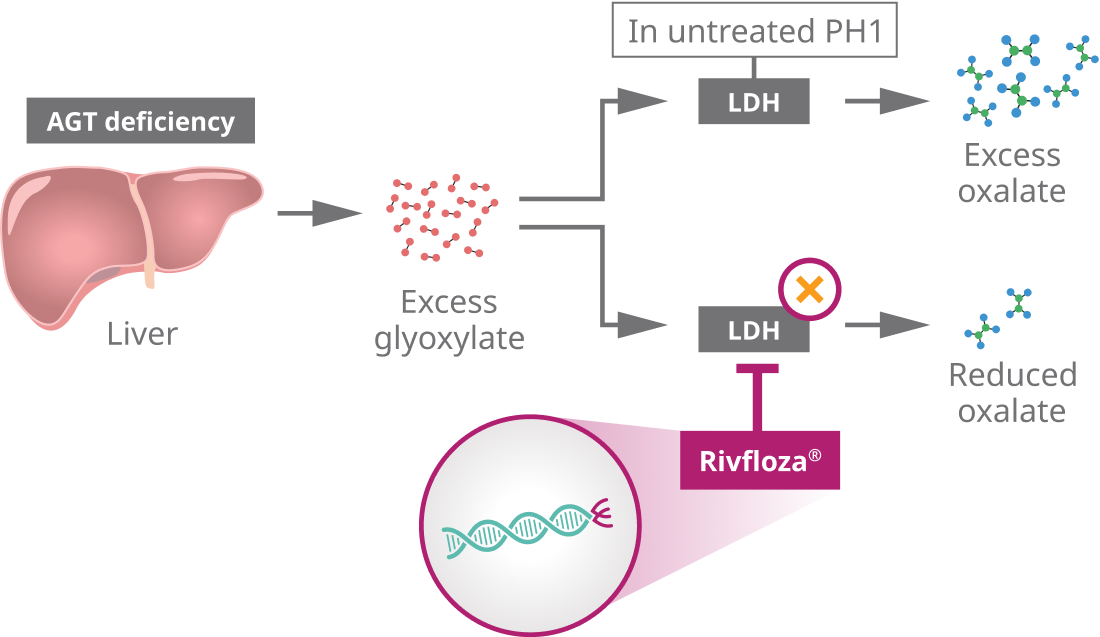
Primary hyperoxaluria type one, or PH1, is a rare genetic disorder in which the overproduction of oxalate in the liver leads to kidney stones and progressive kidney damage. In PH1, a liver enzyme deficiency causes a buildup of glyoxylate, which is converted into excess levels of oxalate by the hepatic enzyme LDH.
Oxalate production can be reduced by selectively targeting and silencing hepatic LDHA. In PH1, glyoxylate is not converted into glycine due to deficiencies in the peroxisomal enzyme AGT. The excess glyoxylate is instead converted to oxalate by hepatic LDH. This excess oxalate in the liver travels to the kidneys, where it binds with calcium to form calcium oxalate crystals, leading to kidney stones and nephrocalcinosis. Deposition of calcium oxalate crystals can lead to progressive kidney damage.
RNAi therapy targets specific messenger RNA to halt the production of disease-causing proteins. Rivfloza®, or nedosiran, injection is an RNAi therapy indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function.
Rivfloza® is engineered using Novo Nordisk’s GalXC™ technology, in which small interfering RNA, or siRNA, is conjugated to GalNAc sugars that selectively bind to ASGPRs on hepatocyte cell surfaces. This design allows Rivfloza® to be delivered to the liver in a specific and targeted manner. Once inside the hepatocyte, Rivfloza® is loaded into the RNA-induced silencing complex, or RISC. The guide strand guides RISC to target messenger RNA strands that contain instructions for making hepatic LDH. RISC cleaves the messenger RNA, inhibiting hepatic LDH production. The selective reduction of hepatic LDH by Rivfloza® through RNA interference reduces oxalate production by the liver, thereby reducing subsequent oxalate burden.
Rivfloza® provides once-monthly, subcutaneous, at-home administration for patients with PH1. Choose once-monthly Rivfloza® for persistent urinary oxalate reduction.
Important Safety Information
Adverse Reactions
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Indication and Usage
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
US25RVZA00051
UOx=urinary oxalate.
Rivfloza® is an RNAi therapy for PH1 that harnesses a natural biological process3
No serious treatment-related adverse events in patients with PH1 who received Rivfloza® in the clinical trials2
Important Safety Information for Rivfloza®
Adverse Reactions
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Please click here for Rivfloza® Prescribing Information.
Important Safety Information for Rivfloza®
Adverse Reactions
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Please click here for Rivfloza® Prescribing Information.
References:
- Groothoff JW, Metry E, Deesker L, et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. 2023;19(3):194-211. doi:10.1038/s41581-022-00661-1
- Rivfloza® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Ariceta G, Barrios K, Brown BD, Hoppe B, Rosskamp R, Langman CB. Hepatic lactate dehydrogenase A: an RNA interference target for the treatment of all known types of primary hyperoxaluria. Kidney Int Rep. 2021;6(4):1088-1098. doi:10.1016/j.ekir.2021.02.029
- Data on file. Novo Nordisk Inc; Plainsboro, NJ.