Product resources library
Browse our library of Rivfloza® product education, including the Prescribing Information, dosing and administration, mechanism of action, and more.
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg
Rivfloza® is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Rivfloza® is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Filter
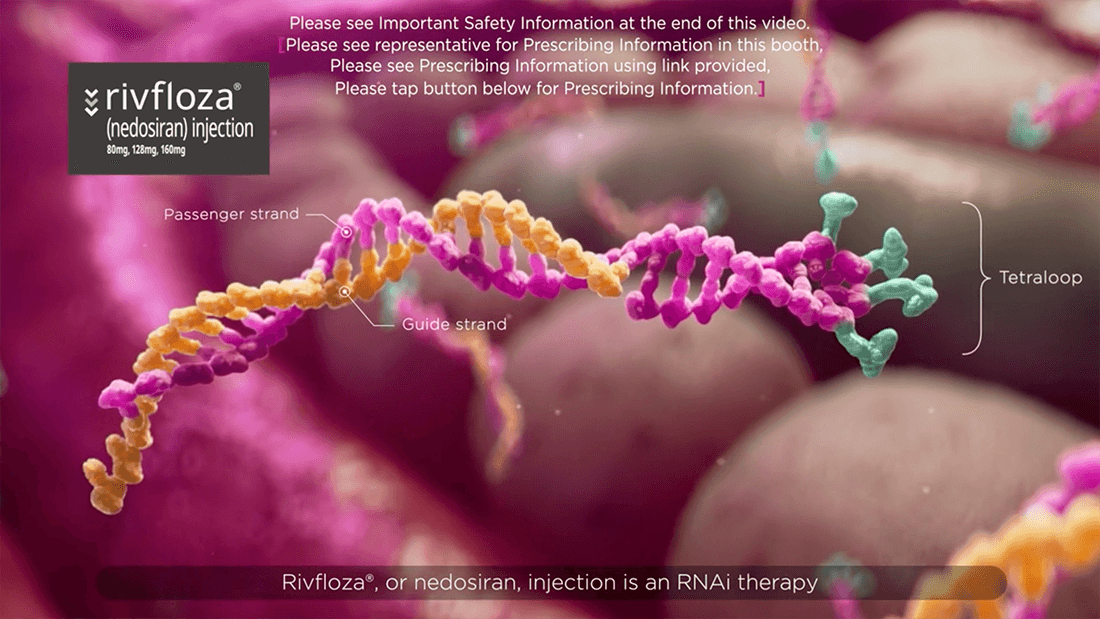
Rivfloza® Mechanism of Action Video
Understand how Rivfloza® works with this animated video.
Important Safety Information for Rivfloza®
Adverse Reactions
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Please click here for Rivfloza® Prescribing Information.
Important Safety Information for Rivfloza®
Adverse Reactions
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Please click here for Rivfloza® Prescribing Information.