For use in adults and children with hemophilia B for on demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes. Not for immune tolerance induction.
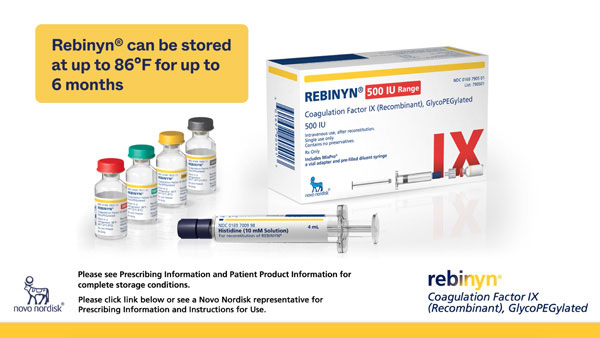
What’s included in the Rebinyn® package:
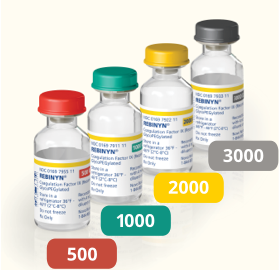
Vial with color-coded cap
Differentiate dose strengths through color-coded vial caps.1
Adapter
Connects the syringe and vial, with a 25-μm inline particle filter.1

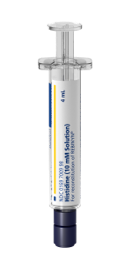
Prefilled diluent syringe
Contains 4 mL of diluent—works with any dose strength.1
Basic reconstitution steps


ATTACH1,a
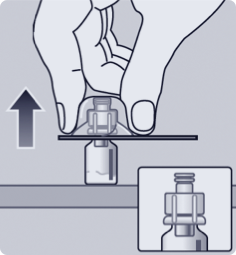

TWIST1,a
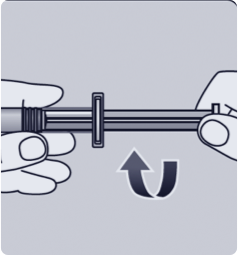
MIX1,a
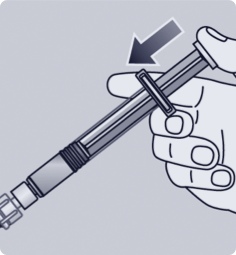

After mixing, the reconstituted solution should be administered within 4 hours.

Preparation
- Always wash hands and ensure that the area is clean before performing the procedures
- Use aseptic technique during the reconstitution procedures
- If the patient uses more than one vial of Rebinyn® per injection, reconstitute each vial according to the instructions
Administration: For intravenous infusion only1


Administer using the following procedure:
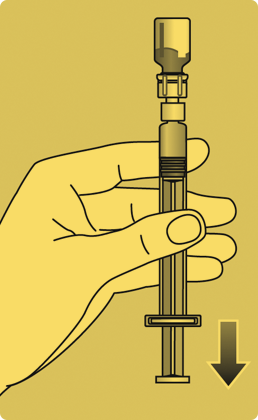
- Invert the Rebinyn® vial and slowly draw the solution into the syringe.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- Attach the syringe to the luer end of an infusion needle set.
- Infuse the reconstituted Rebinyn® intravenously slowly over 1 to 4 minutes.
- After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused Rebinyn®, and other waste materials.
- Accidental needle stick with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. If a needle stick occurs, obtain immediate medical attention. Place needles in a sharps container after single use.1
- Inspect the reconstituted Rebinyn® solution visually prior to administration [see Description (11)]. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed.1
- Do not administer Rebinyn® in the same tubing or container with other medicinal products.1

Caution1:
- The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
- Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer Rebinyn® through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
- If you encounter any problems with attaching the pre-filled histidine-diluent syringe to any Luer-lock compatible device, please contact Novo Nordisk at (844) 303-4448.
Please note, this is not the complete Rebinyn® instructions for use. Please refer to the Instructions for Use included in the Prescribing Information.
Options for ordering
Order Rebinyn® via phone or trading partner. Get help with orders through NovoCare®.
Simple prophylaxis dosing
Convenient, once-weekly doing for all patients.
Important Safety Information for Rebinyn®
Contraindications
- Rebinyn® is contraindicated in patients with a known hypersensitivity to Rebinyn® or its components, including hamster proteins.
Warnings and Precautions
- Hypersensitivity Reactions: Allergic-type hypersensitivity reactions, including anaphylaxis, have occurred with Rebinyn®. Signs may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Discontinue Rebinyn® if allergic- or anaphylactic-type reactions occur and initiate appropriate treatment.
- Inhibitors: The formation of inhibitors (neutralizing antibodies) to Factor IX has occurred following Rebinyn®. If expected plasma factor IX activity levels are not attained, or if bleeding is not controlled as expected with the administered dose, perform an assay that measures Factor IX inhibitor concentration. Monitor all patients using clinical observations and laboratory tests for the development of inhibitors. Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used.
- Thrombotic Events: The use of Factor IX-containing products has been associated with thromboembolic complications. Monitor for thrombotic and consumptive coagulopathy when administering Rebinyn® to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC).
- Nephrotic Syndrome: Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using Rebinyn® for immune tolerance induction have not been established.
Adverse Reactions
- The most common adverse reactions reported in previously treated patients in clinical trials (≥1%) were itching and injection site reactions. The most common adverse reactions (≥1%) in previously untreated patients reported in clinical trials were rash, FIX inhibitors, hypersensitivity, itching, injection site reaction, and anaphylactic reaction.
- Animals administered Rebinyn® showed accumulation of PEG in the choroid plexus, pituitary, circumventricular organs, and cranial motor neurons. The potential clinical implications of these animal findings are unknown. Consider whether the patient is vulnerable to cognitive impairment, such as infants and children who have developing brains, and patients who are cognitively impaired.
Please click here for Rebinyn® Prescribing Information.
Indications and Usage
Rebinyn®, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B (congenital Factor IX deficiency) for on demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of Use: Rebinyn® is not indicated for immune tolerance induction in patients with hemophilia B.
Important Safety Information for Rebinyn®
Contraindications
- Rebinyn® is contraindicated in patients with a known hypersensitivity to Rebinyn® or its components, including hamster proteins.
Warnings and Precautions
- Hypersensitivity Reactions: Allergic-type hypersensitivity reactions, including anaphylaxis, have occurred with Rebinyn®. Signs may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Discontinue Rebinyn® if allergic- or anaphylactic-type reactions occur and initiate appropriate treatment.
- Inhibitors: The formation of inhibitors (neutralizing antibodies) to Factor IX has occurred following Rebinyn®. If expected plasma factor IX activity levels are not attained, or if bleeding is not controlled as expected with the administered dose, perform an assay that measures Factor IX inhibitor concentration. Monitor all patients using clinical observations and laboratory tests for the development of inhibitors. Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used.
- Thrombotic Events: The use of Factor IX-containing products has been associated with thromboembolic complications. Monitor for thrombotic and consumptive coagulopathy when administering Rebinyn® to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC).
- Nephrotic Syndrome: Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using Rebinyn® for immune tolerance induction have not been established.
Adverse Reactions
- The most common adverse reactions reported in previously treated patients in clinical trials (≥1%) were itching and injection site reactions. The most common adverse reactions (≥1%) in previously untreated patients reported in clinical trials were rash, FIX inhibitors, hypersensitivity, itching, injection site reaction, and anaphylactic reaction.
- Animals administered Rebinyn® showed accumulation of PEG in the choroid plexus, pituitary, circumventricular organs, and cranial motor neurons. The potential clinical implications of these animal findings are unknown. Consider whether the patient is vulnerable to cognitive impairment, such as infants and children who have developing brains, and patients who are cognitively impaired.
Please click here for Rebinyn® Prescribing Information.
Indications and Usage
Rebinyn®, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B (congenital Factor IX deficiency) for on demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of Use: Rebinyn® is not indicated for immune tolerance induction in patients with hemophilia B.
Reference:
- Rebinyn [package insert]. Plainsboro, NJ: Novo Nordisk Inc.